Intravital 2-photon imaging of leukocyte trafficking in beating heart
- PMID: 22706307
- PMCID: PMC3386827
- DOI: 10.1172/JCI62970
Intravital 2-photon imaging of leukocyte trafficking in beating heart
Abstract
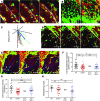
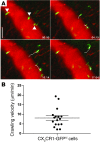
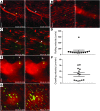
Two-photon intravital microscopy has substantially broadened our understanding of tissue- and organ-specific differences in the regulation of inflammatory responses. However, little is known about the dynamic regulation of leukocyte recruitment into inflamed heart tissue, largely due to technical difficulties inherent in imaging moving tissue. Here, we report a method for imaging beating murine hearts using intravital 2-photon microscopy. Using this method, we visualized neutrophil trafficking at baseline and during inflammation. Ischemia reperfusion injury induced by transplantation or transient coronary artery ligation led to recruitment of neutrophils to the heart, their extravasation from coronary veins, and infiltration of the myocardium where they formed large clusters. Grafting hearts containing mutant ICAM-1, a ligand important for neutrophil recruitment, reduced the crawling velocities of neutrophils within vessels, and markedly inhibited their extravasation. Similar impairment was seen with the inhibition of Mac-1, a receptor for ICAM-1. Blockade of LFA-1, another ICAM-1 receptor, prevented neutrophil adherence to endothelium and extravasation in heart grafts. As inflammatory responses in the heart are of great relevance to public health, this imaging approach holds promise for studying cardiac-specific mechanisms of leukocyte recruitment and identifying novel therapeutic targets for treating heart disease.
Figures
Similar articles
-
Intravital imaging of neutrophil recruitment in hepatic ischemia-reperfusion injury in mice.Transplantation. 2013 Feb 27;95(4):551-8. doi: 10.1097/TP.0b013e31827d62b5. Transplantation. 2013. PMID: 23423266
-
Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation.J Clin Invest. 2019 Feb 26;129(6):2293-2304. doi: 10.1172/JCI126428. J Clin Invest. 2019. PMID: 30830879 Free PMC article.
-
The receptor for activated complement factor 5 (C5aR) conveys myocardial ischemic damage by mediating neutrophil transmigration.Immunobiology. 2013 Sep;218(9):1131-8. doi: 10.1016/j.imbio.2013.03.006. Epub 2013 Mar 28. Immunobiology. 2013. PMID: 23642836
-
Intravital 2-photon imaging, leukocyte trafficking, and the beating heart.Trends Cardiovasc Med. 2013 Nov;23(8):287-93. doi: 10.1016/j.tcm.2013.04.002. Epub 2013 May 22. Trends Cardiovasc Med. 2013. PMID: 23706535 Free PMC article. Review.
-
Intravital Microscopy of the Beating Murine Heart to Understand Cardiac Leukocyte Dynamics.Front Immunol. 2020 Feb 4;11:92. doi: 10.3389/fimmu.2020.00092. eCollection 2020. Front Immunol. 2020. PMID: 32117249 Free PMC article. Review.
Cited by
-
Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal.Cell. 2013 Apr 11;153(2):362-75. doi: 10.1016/j.cell.2013.03.010. Cell. 2013. PMID: 23582326 Free PMC article.
-
Different Roles of Resident and Non-resident Macrophages in Cardiac Fibrosis.Front Cardiovasc Med. 2022 Mar 7;9:818188. doi: 10.3389/fcvm.2022.818188. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35330948 Free PMC article. Review.
-
Anti-LFA-1 induces CD8 T-cell dependent allograft tolerance and augments suppressor phenotype CD8 cells.Cell Immunol. 2018 Oct;332:101-110. doi: 10.1016/j.cellimm.2018.08.003. Epub 2018 Aug 7. Cell Immunol. 2018. PMID: 30103941 Free PMC article.
-
Immune cells in cardiac homeostasis and disease: emerging insights from novel technologies.Eur Heart J. 2022 Apr 19;43(16):1533-1541. doi: 10.1093/eurheartj/ehab842. Eur Heart J. 2022. PMID: 34897403 Free PMC article. Review.
-
Processes of sterile inflammation.J Immunol. 2013 Sep 15;191(6):2857-63. doi: 10.4049/jimmunol.1301539. J Immunol. 2013. PMID: 24014880 Free PMC article. Review.
References
-
- Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol. 2008;180(10):6439–6446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

