Doc2b is a key effector of insulin secretion and skeletal muscle insulin sensitivity
- PMID: 22698913
- PMCID: PMC3447898
- DOI: 10.2337/db11-1525
Doc2b is a key effector of insulin secretion and skeletal muscle insulin sensitivity
Abstract
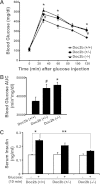
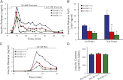
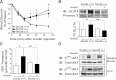
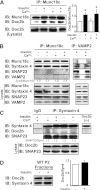
Exocytosis of intracellular vesicles, such as insulin granules, is carried out by soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) and Sec1/Munc18 (SM) proteins. An additional regulatory protein, Doc2b (double C2 domain), has recently been implicated in exocytosis from clonal β-cells and 3T3-L1 adipocytes. Here, we investigated the role of Doc2b in insulin secretion, insulin sensitivity, and the maintenance of whole-body glucose homeostasis. Doc2b heterozygous (Doc2b(+/-)) and homozygous (Doc2b(-/-)) knockout mice exhibited significant whole-body glucose intolerance and peripheral insulin resistance, compared with wild-type littermates. Correspondingly, Doc2b(+/-) and Doc2b(-/-) mice exhibited decreased responsiveness of pancreatic islets to glucose in vivo, with significant attenuation of both phases of insulin secretion ex vivo. Peripheral insulin resistance correlated with ablated insulin-stimulated glucose uptake and GLUT4 vesicle translocation in skeletal muscle from Doc2b-deficient mice, which was coupled to impairments in Munc18c-syntaxin 4 dissociation and in SNARE complex assembly. Hence, Doc2b is a key positive regulator of Munc18c-syntaxin 4-mediated insulin secretion as well as of insulin responsiveness in skeletal muscle, and thus a key effector for glucose homeostasis in vivo. Doc2b's actions in glucose homeostasis may be related to its ability to bind Munc18c and/or directly promote fusion of insulin granules and GLUT4 vesicles in a stimulus-dependent manner.
Figures


Similar articles
-
DOC2B: a novel syntaxin-4 binding protein mediating insulin-regulated GLUT4 vesicle fusion in adipocytes.Diabetes. 2009 Feb;58(2):377-84. doi: 10.2337/db08-0303. Epub 2008 Nov 25. Diabetes. 2009. PMID: 19033398 Free PMC article.
-
Doc2b enrichment enhances glucose homeostasis in mice via potentiation of insulin secretion and peripheral insulin sensitivity.Diabetologia. 2014 Jul;57(7):1476-84. doi: 10.1007/s00125-014-3227-7. Epub 2014 Apr 6. Diabetologia. 2014. PMID: 24705606 Free PMC article.
-
Doc2b serves as a scaffolding platform for concurrent binding of multiple Munc18 isoforms in pancreatic islet β-cells.Biochem J. 2014 Dec 1;464(2):251-8. doi: 10.1042/BJ20140845. Biochem J. 2014. PMID: 25190515 Free PMC article.
-
Exocytosis mechanisms underlying insulin release and glucose uptake: conserved roles for Munc18c and syntaxin 4.Am J Physiol Regul Integr Comp Physiol. 2010 Mar;298(3):R517-31. doi: 10.1152/ajpregu.00597.2009. Epub 2010 Jan 6. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20053958 Free PMC article. Review.
-
Munc18c: a controversial regulator of peripheral insulin action.Trends Endocrinol Metab. 2014 Nov;25(11):601-8. doi: 10.1016/j.tem.2014.06.010. Epub 2014 Jul 12. Trends Endocrinol Metab. 2014. PMID: 25028245 Free PMC article. Review.
Cited by
-
Doc2b Protects β-Cells Against Inflammatory Damage and Enhances Function.Diabetes. 2018 Jul;67(7):1332-1344. doi: 10.2337/db17-1352. Epub 2018 Apr 16. Diabetes. 2018. PMID: 29661782 Free PMC article.
-
Distinct insulin granule subpopulations implicated in the secretory pathology of diabetes types 1 and 2.Elife. 2020 Nov 9;9:e62506. doi: 10.7554/eLife.62506. Elife. 2020. PMID: 33164744 Free PMC article.
-
DOC2b Enhances β-Cell Function via a Novel Tyrosine Phosphorylation-Dependent Mechanism.Diabetes. 2022 Jun 1;71(6):1246-1260. doi: 10.2337/db21-0681. Diabetes. 2022. PMID: 35377441 Free PMC article.
-
Convergent copy number increase of genes associated with freshwater colonization in fishes.Philos Trans R Soc Lond B Biol Sci. 2022 Jul 18;377(1855):20200509. doi: 10.1098/rstb.2020.0509. Epub 2022 May 30. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 35634928 Free PMC article.
-
Promoting Glucose Transporter-4 Vesicle Trafficking along Cytoskeletal Tracks: PAK-Ing Them Out.Front Endocrinol (Lausanne). 2017 Nov 20;8:329. doi: 10.3389/fendo.2017.00329. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 29209279 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

