Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin α4β7
- PMID: 22693444
- PMCID: PMC3364951
- DOI: 10.1371/journal.ppat.1002686
Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin α4β7
Abstract
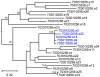
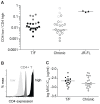
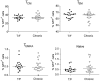
Sexual transmission of human immunodeficiency virus type 1 (HIV-1) most often results from productive infection by a single transmitted/founder (T/F) virus, indicating a stringent mucosal bottleneck. Understanding the viral traits that overcome this bottleneck could have important implications for HIV-1 vaccine design and other prevention strategies. Most T/F viruses use CCR5 to infect target cells and some encode envelope glycoproteins (Envs) that contain fewer potential N-linked glycosylation sites and shorter V1/V2 variable loops than Envs from chronic viruses. Moreover, it has been reported that the gp120 subunits of certain transmitted Envs bind to the gut-homing integrin α4β7, possibly enhancing virus entry and cell-to-cell spread. Here we sought to determine whether subtype C T/F viruses, which are responsible for the majority of new HIV-1 infections worldwide, share biological properties that increase their transmission fitness, including preferential α4β7 engagement. Using single genome amplification, we generated panels of both T/F (n = 20) and chronic (n = 20) Env constructs as well as full-length T/F (n = 6) and chronic (n = 4) infectious molecular clones (IMCs). We found that T/F and chronic control Envs were indistinguishable in the efficiency with which they used CD4 and CCR5. Both groups of Envs also exhibited the same CD4+ T cell subset tropism and showed similar sensitivity to neutralization by CD4 binding site (CD4bs) antibodies. Finally, saturating concentrations of anti-α4β7 antibodies failed to inhibit infection and replication of T/F as well as chronic control viruses, although the growth of the tissue culture-adapted strain SF162 was modestly impaired. These results indicate that the population bottleneck associated with mucosal HIV-1 acquisition is not due to the selection of T/F viruses that use α4β7, CD4 or CCR5 more efficiently.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
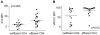


Similar articles
-
HIV-1 envelope, integrins and co-receptor use in mucosal transmission of HIV.J Transl Med. 2011 Jan 27;9 Suppl 1(Suppl 1):S2. doi: 10.1186/1479-5876-9-S1-S2. J Transl Med. 2011. PMID: 21284901 Free PMC article. Review.
-
HIV-1 R5 Macrophage-Tropic Envelope Glycoprotein Trimers Bind CD4 with High Affinity, while the CD4 Binding Site on Non-macrophage-tropic, T-Tropic R5 Envelopes Is Occluded.J Virol. 2018 Jan 2;92(2):e00841-17. doi: 10.1128/JVI.00841-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29118121 Free PMC article.
-
Phenotypic Correlates of HIV-1 Macrophage Tropism.J Virol. 2015 Nov;89(22):11294-311. doi: 10.1128/JVI.00946-15. Epub 2015 Sep 2. J Virol. 2015. PMID: 26339058 Free PMC article.
-
Identification of New Regions in HIV-1 gp120 Variable 2 and 3 Loops that Bind to α4β7 Integrin Receptor.PLoS One. 2015 Dec 1;10(12):e0143895. doi: 10.1371/journal.pone.0143895. eCollection 2015. PLoS One. 2015. PMID: 26625359 Free PMC article. Clinical Trial.
-
Affinofile profiling: how efficiency of CD4/CCR5 usage impacts the biological and pathogenic phenotype of HIV.Virology. 2013 Jan 5;435(1):81-91. doi: 10.1016/j.virol.2012.09.043. Virology. 2013. PMID: 23217618 Free PMC article. Review.
Cited by
-
Efficient single tobamoviral vector-based bioproduction of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 in Nicotiana benthamiana plants and utility of VRC01 in combination microbicides.Antimicrob Agents Chemother. 2013 May;57(5):2076-86. doi: 10.1128/AAC.02588-12. Epub 2013 Feb 12. Antimicrob Agents Chemother. 2013. PMID: 23403432 Free PMC article.
-
Comparison of viral Env proteins from acute and chronic infections with subtype C human immunodeficiency virus type 1 identifies differences in glycosylation and CCR5 utilization and suggests a new strategy for immunogen design.J Virol. 2013 Jul;87(13):7218-33. doi: 10.1128/JVI.03577-12. Epub 2013 Apr 24. J Virol. 2013. PMID: 23616655 Free PMC article.
-
A Small Molecule, Which Competes with MAdCAM-1, Activates Integrin α4β7 and Fails to Prevent Mucosal Transmission of SHIV-SF162P3.PLoS Pathog. 2016 Jun 27;12(6):e1005720. doi: 10.1371/journal.ppat.1005720. eCollection 2016 Jun. PLoS Pathog. 2016. PMID: 27348748 Free PMC article.
-
The puzzling role of CXCR4 in human immunodeficiency virus infection.Theranostics. 2013;3(1):18-25. doi: 10.7150/thno.5392. Epub 2013 Jan 13. Theranostics. 2013. PMID: 23382782 Free PMC article. Review.
-
Advances in the mechanisms and applications of inhibitory oligodeoxynucleotides against immune-mediated inflammatory diseases.Front Pharmacol. 2023 Feb 7;14:1119431. doi: 10.3389/fphar.2023.1119431. eCollection 2023. Front Pharmacol. 2023. PMID: 36825156 Free PMC article. Review.
References
-
- Wolfs TF, Zwart G, Bakker M, Goudsmit J. HIV-1 genomic RNA diversification following sexual and parenteral virus transmission. Virology. 1992;189:103–110. - PubMed
-
- Wolinsky SM, Wike CM, Korber BT, Hutto C, Parks WP, et al. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992;255:1134–1137. - PubMed
-
- Pang S, Shlesinger Y, Daar ES, Moudgil T, Ho DD, et al. Rapid generation of sequence variation during primary HIV-1 infection. AIDS. 1992;6:453–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

