Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-β
- PMID: 22693253
- PMCID: PMC3538149
- DOI: 10.1158/0008-5472.CAN-12-0546
Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-β
Abstract
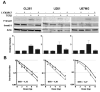
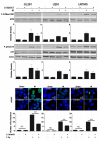
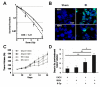
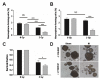
The poor prognosis of glioblastoma (GBM) routinely treated with ionizing radiation (IR) has been attributed to the relative radioresistance of glioma-initiating cells (GIC). Other studies indicate that although GIC are sensitive, the response is mediated by undefined factors in the microenvironment. GBM produce abundant transforming growth factor-β (TGF-β), a pleotropic cytokine that promotes effective DNA damage response. Consistent with this, radiation sensitivity, as measured by clonogenic assay of cultured murine (GL261) and human (U251, U87MG) glioma cell lines, increased by approximately 25% when treated with LY364947, a small-molecule inhibitor of TGF-β type I receptor kinase, before irradiation. Mice bearing GL261 flank tumors treated with 1D11, a pan-isoform TGF-β neutralizing antibody, exhibited significantly increased tumor growth delay following IR. GL261 neurosphere cultures were used to evaluate GIC. LY364947 had no effect on the primary or secondary neurosphere-forming capacity. IR decreased primary neurosphere formation by 28%, but did not reduce secondary neurosphere formation. In contrast, LY364947 treatment before IR decreased primary neurosphere formation by 75% and secondary neurosphere formation by 68%. Notably, GL261 neurospheres produced 3.7-fold more TGF-β per cell compared with conventional culture, suggesting that TGF-β production by GIC promotes effective DNA damage response and self-renewal, which creates microenvironment-mediated resistance. Consistent with this, LY364947 treatment in irradiated GL261 neurosphere-derived cells decreased DNA damage responses, H2AX and p53 phosphorylation, and induction of self-renewal signals, Notch1 and CXCR4. These data motivate the use of TGF-β inhibitors with radiation to improve therapeutic response in patients with GBM.
©2012 AACR.
Figures
Similar articles
-
Blockade of TGF-β signaling by the TGFβR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma.Cancer Res. 2011 Dec 1;71(23):7155-67. doi: 10.1158/0008-5472.CAN-11-1212. Epub 2011 Oct 17. Cancer Res. 2011. PMID: 22006998
-
Attenuation of the DNA damage response by transforming growth factor-beta inhibitors enhances radiation sensitivity of non-small-cell lung cancer cells in vitro and in vivo.Int J Radiat Oncol Biol Phys. 2015 Jan 1;91(1):91-9. doi: 10.1016/j.ijrobp.2014.09.026. Int J Radiat Oncol Biol Phys. 2015. PMID: 25835621
-
TGFβ1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo.Clin Cancer Res. 2011 Nov 1;17(21):6754-65. doi: 10.1158/1078-0432.CCR-11-0544. Epub 2011 Oct 25. Clin Cancer Res. 2011. PMID: 22028490 Free PMC article.
-
Brain tumor stem cells: Molecular characteristics and their impact on therapy.Mol Aspects Med. 2014 Oct;39:82-101. doi: 10.1016/j.mam.2013.06.004. Epub 2013 Jul 4. Mol Aspects Med. 2014. PMID: 23831316 Free PMC article. Review.
-
[Cancer stem cells, cornerstone of radioresistance and perspectives for radiosensitization: glioblastoma as an example].Bull Cancer. 2012 Dec;99(12):1153-60. doi: 10.1684/bdc.2012.1666. Bull Cancer. 2012. PMID: 23228708 Review. French.
Cited by
-
Cytokine Modification of Adoptive Chimeric Antigen Receptor Immunotherapy for Glioblastoma.Cancers (Basel). 2023 Dec 15;15(24):5852. doi: 10.3390/cancers15245852. Cancers (Basel). 2023. PMID: 38136398 Free PMC article. Review.
-
The Role of Cytokines and Chemokines in Shaping the Immune Microenvironment of Glioblastoma: Implications for Immunotherapy.Cells. 2021 Mar 9;10(3):607. doi: 10.3390/cells10030607. Cells. 2021. PMID: 33803414 Free PMC article. Review.
-
Hitting Them Where They Live: Targeting the Glioblastoma Perivascular Stem Cell Niche.Curr Pathobiol Rep. 2013 Jun 1;1(2):101-110. doi: 10.1007/s40139-013-0012-0. Curr Pathobiol Rep. 2013. PMID: 23766946 Free PMC article.
-
A Systematic Comparison Identifies an ATP-Based Viability Assay as Most Suitable Read-Out for Drug Screening in Glioma Stem-Like Cells.Stem Cells Int. 2016;2016:5623235. doi: 10.1155/2016/5623235. Epub 2016 May 5. Stem Cells Int. 2016. PMID: 27274737 Free PMC article.
-
Interleukins in glioblastoma pathophysiology: implications for therapy.Br J Pharmacol. 2013 Feb;168(3):591-606. doi: 10.1111/bph.12008. Br J Pharmacol. 2013. PMID: 23062197 Free PMC article. Review.
References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. - PubMed
-
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. - PubMed
-
- Rosenblum ML, Gerosa M, Dougherty DV, Reese C, Barger GR, Davis RL, et al. Age-related chemosensitivity of stem cells from human malignant brain tumours. Lancet. 1982;1:885–7. - PubMed
-
- Frosina G. DNA repair and resistance of gliomas to chemotherapy and radiotherapy. Mol Cancer Res. 2009;7:989–99. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

