Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects
- PMID: 22683779
- PMCID: PMC3438344
- DOI: 10.1038/nm.2834
Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects
Abstract
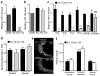
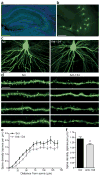
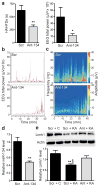
Temporal lobe epilepsy is a common, chronic neurological disorder characterized by recurrent spontaneous seizures. MicroRNAs (miRNAs) are small, noncoding RNAs that regulate post-transcriptional expression of protein-coding mRNAs, which may have key roles in the pathogenesis of neurological disorders. In experimental models of prolonged, injurious seizures (status epilepticus) and in human epilepsy, we found upregulation of miR-134, a brain-specific, activity-regulated miRNA that has been implicated in the control of dendritic spine morphology. Silencing of miR-134 expression in vivo using antagomirs reduced hippocampal CA3 pyramidal neuron dendrite spine density by 21% and rendered mice refractory to seizures and hippocampal injury caused by status epilepticus. Depletion of miR-134 after status epilepticus in mice reduced the later occurrence of spontaneous seizures by over 90% and mitigated the attendant pathological features of temporal lobe epilepsy. Thus, silencing miR-134 exerts prolonged seizure-suppressant and neuroprotective actions; determining whether these are anticonvulsant effects or are truly antiepileptogenic effects requires additional experimentation.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures



Similar articles
-
Antagomirs targeting microRNA-134 increase hippocampal pyramidal neuron spine volume in vivo and protect against pilocarpine-induced status epilepticus.Brain Struct Funct. 2015 Jul;220(4):2387-99. doi: 10.1007/s00429-014-0798-5. Epub 2014 May 30. Brain Struct Funct. 2015. PMID: 24874920
-
Antagonizing Increased miR-135a Levels at the Chronic Stage of Experimental TLE Reduces Spontaneous Recurrent Seizures.J Neurosci. 2019 Jun 26;39(26):5064-5079. doi: 10.1523/JNEUROSCI.3014-18.2019. Epub 2019 Apr 23. J Neurosci. 2019. PMID: 31015341 Free PMC article.
-
MicroRNA-23a contributes to hippocampal neuronal injuries and spatial memory impairment in an experimental model of temporal lobe epilepsy.Brain Res Bull. 2019 Oct;152:175-183. doi: 10.1016/j.brainresbull.2019.07.021. Epub 2019 Jul 20. Brain Res Bull. 2019. PMID: 31336125
-
Epilepsy and microRNA.Neuroscience. 2013 May 15;238:218-29. doi: 10.1016/j.neuroscience.2013.02.027. Epub 2013 Feb 26. Neuroscience. 2013. PMID: 23485811 Review.
-
Antagomirs and microRNA in status epilepticus.Epilepsia. 2013 Sep;54 Suppl 6(0 6):17-9. doi: 10.1111/epi.12267. Epilepsia. 2013. PMID: 24001063 Free PMC article. Review.
Cited by
-
Role of MicroRNA in Governing Synaptic Plasticity.Neural Plast. 2016;2016:4959523. doi: 10.1155/2016/4959523. Epub 2016 Mar 13. Neural Plast. 2016. PMID: 27034846 Free PMC article. Review.
-
Immunoproteasome deficiency results in age-dependent development of epilepsy.Brain Commun. 2024 Jan 29;6(1):fcae017. doi: 10.1093/braincomms/fcae017. eCollection 2024. Brain Commun. 2024. PMID: 38317856 Free PMC article.
-
Neuroinvasive Listeria monocytogenes infection triggers accumulation of brain CD8+ tissue-resident memory T cells in a miR-155-dependent fashion.J Neuroinflammation. 2020 Sep 2;17(1):259. doi: 10.1186/s12974-020-01929-8. J Neuroinflammation. 2020. PMID: 32878636 Free PMC article.
-
MicroRNAs of the miR379-410 cluster: New players in embryonic neurogenesis and regulators of neuronal function.Neurogenesis (Austin). 2015 Mar 4;2(1):e1004970. doi: 10.1080/23262133.2015.1004970. eCollection 2015. Neurogenesis (Austin). 2015. PMID: 27504472 Free PMC article. Review.
-
Adult-specific functions of animal microRNAs.Nat Rev Genet. 2013 Aug;14(8):535-48. doi: 10.1038/nrg3471. Epub 2013 Jul 2. Nat Rev Genet. 2013. PMID: 23817310 Free PMC article. Review.
References
-
- Pitkanen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10:173–186. - PubMed
-
- DeFelipe J. Chandelier cells and epilepsy. Brain. 1999;122:1807–1822. - PubMed
-
- McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE. 2006;2006:re12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

