Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination
- PMID: 22682227
- PMCID: PMC3372862
- DOI: 10.1016/j.cmet.2012.03.018
Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination
Abstract
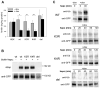
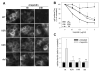
Ferroportin exports iron into plasma from absorptive enterocytes, erythrophagocytosing macrophages, and hepatic stores. The hormone hepcidin controls cellular iron export and plasma iron concentrations by binding to ferroportin and causing its internalization and degradation. We explored the mechanism of hepcidin-induced endocytosis of ferroportin, the key molecular event in systemic iron homeostasis. Hepcidin binding caused rapid ubiquitination of ferroportin in cell lines overexpressing ferroportin and in murine bone marrow-derived macrophages. No hepcidin-dependent ubiquitination was observed in C326S ferroportin mutant which does not bind hepcidin. Substitutions of lysines between residues 229 and 269 in the third cytoplasmic loop of ferroportin prevented hepcidin-dependent ubiquitination and endocytosis of ferroportin, and promoted cellular iron export even in the presence of hepcidin. The human ferroportin mutation K240E, previously associated with clinical iron overload, caused hepcidin resistance in vitro by interfering with ferroportin ubiquitination. Our study demonstrates that ubiquitination is the functionally relevant signal for hepcidin-induced ferroportin endocytosis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
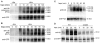

Similar articles
-
Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin.Blood. 2018 Feb 22;131(8):899-910. doi: 10.1182/blood-2017-05-786590. Epub 2017 Dec 13. Blood. 2018. PMID: 29237594 Free PMC article.
-
High-throughput screening of small molecules identifies hepcidin antagonists.Mol Pharmacol. 2013 Mar;83(3):681-90. doi: 10.1124/mol.112.083428. Epub 2013 Jan 4. Mol Pharmacol. 2013. PMID: 23292796 Free PMC article.
-
Hepcidin and ferroportin: the new players in iron metabolism.Semin Liver Dis. 2011 Aug;31(3):272-9. doi: 10.1055/s-0031-1286058. Epub 2011 Sep 7. Semin Liver Dis. 2011. PMID: 21901657 Free PMC article. Review.
-
Hepcidin targets ferroportin for degradation in hepatocytes.Haematologica. 2010 Mar;95(3):501-4. doi: 10.3324/haematol.2009.014399. Epub 2009 Sep 22. Haematologica. 2010. PMID: 19773263 Free PMC article.
-
The molecular basis of iron overload disorders and iron-linked anemias.Int J Hematol. 2011 Jan;93(1):14-20. doi: 10.1007/s12185-010-0760-0. Epub 2011 Jan 7. Int J Hematol. 2011. PMID: 21210258 Review.
Cited by
-
Reduced sensitivity of the ferroportin Q248H mutant to physiological concentrations of hepcidin.Haematologica. 2013 Mar;98(3):455-63. doi: 10.3324/haematol.2012.066530. Epub 2012 Oct 12. Haematologica. 2013. PMID: 23065513 Free PMC article.
-
Serum testosterone levels and excessive erythrocytosis during the process of adaptation to high altitudes.Asian J Androl. 2013 May;15(3):368-74. doi: 10.1038/aja.2012.170. Epub 2013 Mar 25. Asian J Androl. 2013. PMID: 23524530 Free PMC article. Review.
-
Anemia in inflammatory bowel disease: an under-estimated problem?Front Med (Lausanne). 2015 Jan 19;1:58. doi: 10.3389/fmed.2014.00058. eCollection 2014. Front Med (Lausanne). 2015. PMID: 25646159 Free PMC article. Review.
-
In vitro reconstitution of transition metal transporters.J Biol Chem. 2024 Aug;300(8):107589. doi: 10.1016/j.jbc.2024.107589. Epub 2024 Jul 19. J Biol Chem. 2024. PMID: 39032653 Free PMC article. Review.
-
Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function.Proc Natl Acad Sci U S A. 2015 Mar 10;112(10):3164-9. doi: 10.1073/pnas.1422373112. Epub 2015 Feb 23. Proc Natl Acad Sci U S A. 2015. PMID: 25713362 Free PMC article.
References
-
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. - PubMed
-
- Cremonesi L, Forni GL, Soriani N, Lamagna M, Fermo I, Daraio F, Galli A, Pietra D, Malcovati L, Ferrari M, Camaschella C, Cazzola M. Genetic and clinical heterogeneity of ferroportin disease. Br J Haematol. 2005;131:663–670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

