Invasive breast cancer induces laminin-332 upregulation and integrin β4 neoexpression in myofibroblasts to confer an anoikis-resistant phenotype during tissue remodeling
- PMID: 22673183
- PMCID: PMC3446351
- DOI: 10.1186/bcr3203
Invasive breast cancer induces laminin-332 upregulation and integrin β4 neoexpression in myofibroblasts to confer an anoikis-resistant phenotype during tissue remodeling
Abstract
Introduction: Although development of anoikis-resistant myofibroblasts during tissue remodeling is known to be associated with tumor invasion, the mechanism by which myofibroblasts become resistant to anoikis is unknown. We previously demonstrated laminin-332 upregulation in the fibrosis around invasive ductal carcinoma (IDC). Because laminin-332 promotes cell survival through binding to integrins, we hypothesized that invasive breast cancer cells confer an anoikis-resistant phenotype on myofibroblasts by upregulating laminin-332 expression during tissue remodeling. Here, we demonstrate that invasive breast cancer cells induce laminin-332 upregulation and integrin β4 neoexpression in myofibroblasts to confer an anoikis-resistant phenotype.
Methods: Three types of fibroblasts were isolated from the tumor burden, the fibrosis, and normal tissue of patients with early stage IDC (less than 10 mm diameter), designated cancer-associated fibroblasts (CAFs), interface fibroblasts (InFs), and normal breast fibroblasts (NBFs), respectively. To investigate direct and indirect crosstalk with tumor cells, fibroblasts were co-cultured with invasive MDA-MB-231 or noninvasive MCF7 cells or in conditioned medium. Anoikis resistance of fibroblasts was measured by cell viability and caspase-3 activity after incubation on poly-HEMA coated plates for 72 hours. Involvement of laminin-332/integrin α3β1 or α6β4 signaling in anoikis resistance was confirmed by treatment with purified laminin-332 or blocking antibodies against laminin-332, integrin β1, or integrin β4.
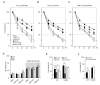
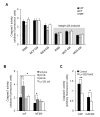
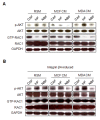
Results: MDA-MB-231 cells induced laminin-332 upregulation and integrin β4 neoexpression in fibroblasts, leading to anoikis resistance. InFs showed a higher endogenous level of laminin-332 than did CAFs and NBFs. After stimulation with MDA-MB-231-conditioned medium, laminin-332 expression of InFs was dramatically increased and maintained under anoikis conditions. Laminin-332 upregulation was also observed in CAFs and NBFs, but at a lower level than in InFs. Laminin-332 induced Akt (Ser473) phosphorylation by binding to integrin α3β1. Integrin β4 neoexpression induced laminin-332-independent Rac1 activation and promoted anoikis resistance in fibroblasts approximately twofold more effectively than did laminin-332, regardless of the type of fibroblast. In addition, integrin β4 expression suppressed fibroblast aggregation in conditions of anoikis.
Conclusion: Invasive breast cancer cells confer an anoikis-resistant phenotype on myofibroblasts during tissue remodeling by inducing laminin-332 upregulation and integrin β4 neoexpression. Interface fibroblasts appear to be the primary myofibroblasts that interact with invasive tumor cells during tissue remodeling.
Figures




Similar articles
-
Anoikis resistance--protagonists of breast cancer cells survive and metastasize after ECM detachment.Cell Commun Signal. 2023 Aug 3;21(1):190. doi: 10.1186/s12964-023-01183-4. Cell Commun Signal. 2023. PMID: 37537585 Free PMC article. Review.
-
Loss of beta4 integrin subunit reduces the tumorigenicity of MCF7 mammary cells and causes apoptosis upon hormone deprivation.Clin Cancer Res. 2006 Jun 1;12(11 Pt 1):3280-7. doi: 10.1158/1078-0432.CCR-05-2223. Clin Cancer Res. 2006. PMID: 16740748
-
Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts.Cell Signal. 2007 Apr;19(4):761-71. doi: 10.1016/j.cellsig.2006.10.001. Epub 2006 Nov 17. Cell Signal. 2007. PMID: 17113264 Free PMC article.
-
miR-221/222 control luminal breast cancer tumor progression by regulating different targets.Cell Cycle. 2014;13(11):1811-26. doi: 10.4161/cc.28758. Epub 2014 Apr 15. Cell Cycle. 2014. PMID: 24736554 Free PMC article.
-
Integrin α3β1 as a breast cancer target.Expert Opin Ther Targets. 2011 Oct;15(10):1197-210. doi: 10.1517/14728222.2011.609557. Epub 2011 Aug 13. Expert Opin Ther Targets. 2011. PMID: 21838596 Free PMC article. Review.
Cited by
-
Extracellular Matrix: Emerging Roles and Potential Therapeutic Targets for Breast Cancer.Front Oncol. 2021 Apr 22;11:650453. doi: 10.3389/fonc.2021.650453. eCollection 2021. Front Oncol. 2021. PMID: 33968752 Free PMC article. Review.
-
Anoikis resistance--protagonists of breast cancer cells survive and metastasize after ECM detachment.Cell Commun Signal. 2023 Aug 3;21(1):190. doi: 10.1186/s12964-023-01183-4. Cell Commun Signal. 2023. PMID: 37537585 Free PMC article. Review.
-
The Interaction between Laminin-332 and α3β1 Integrin Determines Differentiation and Maintenance of CAFs, and Supports Invasion of Pancreatic Duct Adenocarcinoma Cells.Cancers (Basel). 2018 Dec 21;11(1):14. doi: 10.3390/cancers11010014. Cancers (Basel). 2018. PMID: 30583482 Free PMC article.
-
Risk assessment, disease prevention and personalised treatments in breast cancer: is clinically qualified integrative approach in the horizon?EPMA J. 2013 Feb 19;4(1):6. doi: 10.1186/1878-5085-4-6. EPMA J. 2013. PMID: 23418957 Free PMC article.
-
The G Protein-Coupled Estrogen Receptor (GPER) Expression Correlates with Pro-Metastatic Pathways in ER-Negative Breast Cancer: A Bioinformatics Analysis.Cells. 2020 Mar 4;9(3):622. doi: 10.3390/cells9030622. Cells. 2020. PMID: 32143514 Free PMC article.
References
-
- Maher TM, Evans IC, Bottoms SE, Mercer PF, Thorley AJ, Nicholson AG, Laurent GJ, Tetley TD, Chambers RC, McAnulty RJ. Diminished prostaglandin E2 contributes to the apoptosis paradox in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182:73–82. doi: 10.1164/rccm.200905-0674OC. - DOI - PMC - PubMed
-
- Uhal BD. Apoptosis in lung fibrosis and repair. Chest. 2002;122:(6 Suppl):293S–298S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

