Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin
- PMID: 22665765
- PMCID: PMC3382560
- DOI: 10.1073/pnas.1205726109
Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin
Abstract
Casparian strips are ring-like cell-wall modifications in the root endodermis of vascular plants. Their presence generates a paracellular barrier, analogous to animal tight junctions, that is thought to be crucial for selective nutrient uptake, exclusion of pathogens, and many other processes. Despite their importance, the chemical nature of Casparian strips has remained a matter of debate, confounding further molecular analysis. Suberin, lignin, lignin-like polymers, or both, have been claimed to make up Casparian strips. Here we show that, in Arabidopsis, suberin is produced much too late to take part in Casparian strip formation. In addition, we have generated plants devoid of any detectable suberin, which still establish functional Casparian strips. In contrast, manipulating lignin biosynthesis abrogates Casparian strip formation. Finally, monolignol feeding and lignin-specific chemical analysis indicates the presence of archetypal lignin in Casparian strips. Our findings establish the chemical nature of the primary root-diffusion barrier in Arabidopsis and enable a mechanistic dissection of the formation of Casparian strips, which are an independent way of generating tight junctions in eukaryotes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



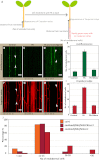
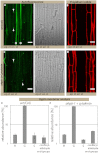
Similar articles
-
In vivo cytological and chemical analysis of Casparian strips using stimulated Raman scattering microscopy.J Plant Physiol. 2018 Jan;220:136-144. doi: 10.1016/j.jplph.2017.11.002. Epub 2017 Nov 14. J Plant Physiol. 2018. PMID: 29175545
-
Role of LOTR1 in Nutrient Transport through Organization of Spatial Distribution of Root Endodermal Barriers.Curr Biol. 2017 Mar 6;27(5):758-765. doi: 10.1016/j.cub.2017.01.030. Epub 2017 Feb 23. Curr Biol. 2017. PMID: 28238658
-
The MYB36 transcription factor orchestrates Casparian strip formation.Proc Natl Acad Sci U S A. 2015 Aug 18;112(33):10533-8. doi: 10.1073/pnas.1507691112. Epub 2015 Jun 29. Proc Natl Acad Sci U S A. 2015. PMID: 26124109 Free PMC article.
-
Building and breaking of a barrier: Suberin plasticity and function in the endodermis.Curr Opin Plant Biol. 2021 Dec;64:102153. doi: 10.1016/j.pbi.2021.102153. Epub 2021 Nov 30. Curr Opin Plant Biol. 2021. PMID: 34861611 Review.
-
Lignin developmental patterns and Casparian strip as apoplastic barriers: A review.Int J Biol Macromol. 2024 Mar;260(Pt 2):129595. doi: 10.1016/j.ijbiomac.2024.129595. Epub 2024 Jan 20. Int J Biol Macromol. 2024. PMID: 38253138 Review.
Cited by
-
A protocol combining multiphoton microscopy and propidium iodide for deep 3D root meristem imaging in rice: application for the screening and identification of tissue-specific enhancer trap lines.Plant Methods. 2018 Oct 29;14:96. doi: 10.1186/s13007-018-0364-x. eCollection 2018. Plant Methods. 2018. PMID: 30386414 Free PMC article.
-
Visualizing polymeric components that define distinct root barriers across plant lineages.Development. 2021 Dec 1;148(23):dev199820. doi: 10.1242/dev.199820. Epub 2021 Dec 8. Development. 2021. PMID: 34878124 Free PMC article.
-
Phosphorylated B6 vitamer deficiency in SALT OVERLY SENSITIVE 4 mutants compromises shoot and root development.Plant Physiol. 2022 Jan 20;188(1):220-240. doi: 10.1093/plphys/kiab475. Plant Physiol. 2022. PMID: 34730814 Free PMC article.
-
A Missing Link in Radial Ion Transport: Ion Transporters in the Endodermis.Front Plant Sci. 2019 Jun 4;10:713. doi: 10.3389/fpls.2019.00713. eCollection 2019. Front Plant Sci. 2019. PMID: 31231406 Free PMC article.
-
ABCG transporters are required for suberin and pollen wall extracellular barriers in Arabidopsis.Plant Cell. 2014 Sep;26(9):3569-88. doi: 10.1105/tpc.114.129049. Epub 2014 Sep 12. Plant Cell. 2014. PMID: 25217507 Free PMC article.
References
-
- Roppolo D, et al. A novel protein family mediates Casparian strip formation in the endodermis. Nature. 2011;473:380–383. - PubMed
-
- Caspary R. Remarks on the protective sheath and the formation of stem and root (translated from German) Jahrbücher für wissenschaftliche Botanik. 1865;4:101–124.
-
- Vanfleet DS. Histochemistry and function of the endodermis. Bot Rev. 1961;27:165–220.
-
- Taiz L, Zeiger E. Plant Physiology. 4th Ed. Sunderland, MA: Sinauer Associates; 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

