Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: a report from the children's oncology group
- PMID: 22665540
- PMCID: PMC3397787
- DOI: 10.1200/JCO.2011.37.4546
Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: a report from the children's oncology group
Abstract
Purpose: Despite efforts to intensify chemotherapy, survival for patients with metastatic osteosarcoma remains poor. Overexpression of human epidermal growth factor receptor 2 (HER2) in osteosarcoma has been shown to predict poor therapeutic response and decreased survival. This study tests the safety and feasibility of delivering biologically targeted therapy by combining trastuzumab with standard chemotherapy in patients with metastatic osteosarcoma and HER2 overexpression.
Patients and methods: Among 96 evaluable patients with newly diagnosed metastatic osteosarcoma, 41 had tumors that were HER2-positive by immunohistochemistry. All patients received chemotherapy with cisplatin, doxorubicin, methotrexate, ifosfamide, and etoposide. Dexrazoxane was administered with doxorubicin to minimize the risk of cardiotoxicity from treatment with trastuzumab and anthracycline. Only patients with HER2 overexpression received concurrent therapy with trastuzumab given for 34 consecutive weeks.
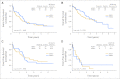
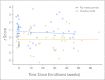
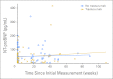
Results: The 30-month event-free and overall survival rates for patients with HER2 overexpression treated with chemotherapy and trastuzumab were 32% and 59%, respectively. For patients without HER2 overexpression, treated with chemotherapy alone, the 30-month event-free and overall survival rates were 32% and 50%, respectively. There was no clinically significant short-term cardiotoxicity in patients treated with trastuzumab and doxorubicin.
Conclusion: Despite intensive chemotherapy plus trastuzumab for patients with HER2-positive disease, the outcome for all patients was poor, with no significant difference between the HER2-positive and HER2-negative groups. Although our findings suggest that trastuzumab can be safely delivered in combination with anthracycline-based chemotherapy and dexrazoxane, its therapeutic benefit remains uncertain. Definitive assessment of trastuzumab's potential role in treating osteosarcoma would require a randomized study of patients with HER2-positive disease.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Similar articles
-
HERMIONE: a randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician's choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer.BMC Cancer. 2016 Jun 3;16:352. doi: 10.1186/s12885-016-2385-z. BMC Cancer. 2016. PMID: 27259714 Free PMC article. Clinical Trial.
-
Intensified Chemotherapy With Dexrazoxane Cardioprotection in Newly Diagnosed Nonmetastatic Osteosarcoma: A Report From the Children's Oncology Group.Pediatr Blood Cancer. 2016 Jan;63(1):54-61. doi: 10.1002/pbc.25753. Epub 2015 Sep 23. Pediatr Blood Cancer. 2016. PMID: 26398490 Free PMC article.
-
Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2.N Engl J Med. 2001 Mar 15;344(11):783-92. doi: 10.1056/NEJM200103153441101. N Engl J Med. 2001. PMID: 11248153 Clinical Trial.
-
Anthracycline-trastuzumab regimens for HER2/neu-overexpressing breast cancer: current experience and future strategies.Ann Oncol. 2008 Sep;19(9):1530-9. doi: 10.1093/annonc/mdn292. Epub 2008 May 13. Ann Oncol. 2008. PMID: 18480068 Review.
-
A systematic review of dual targeting in HER2-positive breast cancer.Cancer Treat Rev. 2014 Mar;40(2):259-70. doi: 10.1016/j.ctrv.2013.09.002. Epub 2013 Sep 11. Cancer Treat Rev. 2014. PMID: 24080156 Review.
Cited by
-
Effects of microenvironment in osteosarcoma on chemoresistance and the promise of immunotherapy as an osteosarcoma therapeutic modality.Front Immunol. 2022 Oct 13;13:871076. doi: 10.3389/fimmu.2022.871076. eCollection 2022. Front Immunol. 2022. PMID: 36311748 Free PMC article. Review.
-
HER-2 expression in biopsy and surgical specimen on prognosis of osteosarcoma: A systematic review and meta-analysis of 16 studies.Medicine (Baltimore). 2016 Jun;95(23):e3661. doi: 10.1097/MD.0000000000003661. Medicine (Baltimore). 2016. PMID: 27281068 Free PMC article. Review.
-
Research models and mesenchymal/epithelial plasticity of osteosarcoma.Cell Biosci. 2021 May 22;11(1):94. doi: 10.1186/s13578-021-00600-w. Cell Biosci. 2021. PMID: 34022967 Free PMC article. Review.
-
Targeted therapy for osteosarcoma: a review.J Cancer Res Clin Oncol. 2023 Aug;149(9):6785-6797. doi: 10.1007/s00432-023-04614-4. Epub 2023 Feb 18. J Cancer Res Clin Oncol. 2023. PMID: 36807762 Review.
-
Innate Immune Cells: A Potential and Promising Cell Population for Treating Osteosarcoma.Front Immunol. 2019 May 16;10:1114. doi: 10.3389/fimmu.2019.01114. eCollection 2019. Front Immunol. 2019. PMID: 31156651 Free PMC article. Review.
References
-
- Lewis DR, Ries G. Cancers of the bone and joint. SEER Survival Monograph. http://seer.cancer.gov/publications/survival/surv_bone_joint.pdf.
-
- Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteosarcoma: The Memorial Sloan Kettering experience. J Clin Oncol. 1992;10:5–15. - PubMed
-
- Harris MB, Gieser P, Goorin AM, et al. Treatment of metastatic osteosarcoma at diagnosis: A Pediatric Oncology Group study. J Clin Oncol. 1998;16:3641–3648. - PubMed
-
- Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. - PubMed
-
- Kager L, Zoubeck A, Potschger U, et al. Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2003;21:2011–2018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

