F-actin polymerization and retrograde flow drive sustained PLCγ1 signaling during T cell activation
- PMID: 22665519
- PMCID: PMC3373411
- DOI: 10.1083/jcb.201201018
F-actin polymerization and retrograde flow drive sustained PLCγ1 signaling during T cell activation
Abstract
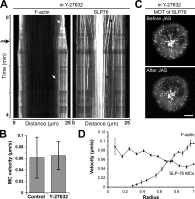
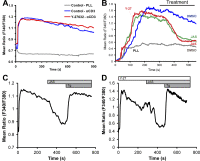
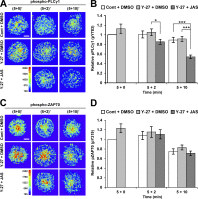
Activation of T cells by antigen-presenting cells involves assembly of signaling molecules into dynamic microclusters (MCs) within a specialized membrane domain termed the immunological synapse (IS). Actin and myosin IIA localize to the IS, and depletion of F-actin abrogates MC movement and T cell activation. However, the mechanisms that coordinate actomyosin dynamics and T cell receptor signaling are poorly understood. Using pharmacological inhibitors that perturb individual aspects of actomyosin dynamics without disassembling the network, we demonstrate that F-actin polymerization is the primary driver of actin retrograde flow, whereas myosin IIA promotes long-term integrity of the IS. Disruption of F-actin retrograde flow, but not myosin IIA contraction, arrested MC centralization and inhibited sustained Ca(2+) signaling at the level of endoplasmic reticulum store release. Furthermore, perturbation of retrograde flow inhibited PLCγ1 phosphorylation within MCs but left Zap70 activity intact. These studies highlight the importance of ongoing actin polymerization as a central driver of actomyosin retrograde flow, MC centralization, and sustained Ca(2+) signaling.
Figures
Similar articles
-
Actin retrograde flow and actomyosin II arc contraction drive receptor cluster dynamics at the immunological synapse in Jurkat T cells.Mol Biol Cell. 2012 Mar;23(5):834-52. doi: 10.1091/mbc.E11-08-0731. Epub 2012 Jan 4. Mol Biol Cell. 2012. PMID: 22219382 Free PMC article.
-
Hematopoietic lineage cell-specific protein 1 is recruited to the immunological synapse by IL-2-inducible T cell kinase and regulates phospholipase Cgamma1 Microcluster dynamics during T cell spreading.J Immunol. 2009 Dec 1;183(11):7352-61. doi: 10.4049/jimmunol.0900973. Epub 2009 Nov 16. J Immunol. 2009. PMID: 19917685 Free PMC article.
-
T cell antigen receptor signaling and immunological synapse stability require myosin IIA.Nat Immunol. 2009 May;10(5):531-9. doi: 10.1038/ni.1723. Epub 2009 Apr 6. Nat Immunol. 2009. PMID: 19349987 Free PMC article.
-
Coordinate control of cytoskeletal remodeling and calcium mobilization during T-cell activation.Immunol Rev. 2013 Nov;256(1):80-94. doi: 10.1111/imr.12123. Immunol Rev. 2013. PMID: 24117814 Free PMC article. Review.
-
Spatio-temporal image correlation spectroscopy and super-resolution microscopy to quantify molecular dynamics in T cells.Methods. 2018 May 1;140-141:112-118. doi: 10.1016/j.ymeth.2018.01.017. Epub 2018 Feb 2. Methods. 2018. PMID: 29410223 Review.
Cited by
-
A tug of war between filament treadmilling and myosin induced contractility generates actin rings.Elife. 2022 Oct 21;11:e82658. doi: 10.7554/eLife.82658. Elife. 2022. PMID: 36269229 Free PMC article.
-
VAMP7 controls T cell activation by regulating the recruitment and phosphorylation of vesicular Lat at TCR-activation sites.Nat Immunol. 2013 Jul;14(7):723-31. doi: 10.1038/ni.2609. Epub 2013 May 12. Nat Immunol. 2013. PMID: 23666293
-
T cell antigen receptor activation and actin cytoskeleton remodeling.Biochim Biophys Acta. 2014 Feb;1838(2):546-56. doi: 10.1016/j.bbamem.2013.05.004. Epub 2013 May 14. Biochim Biophys Acta. 2014. PMID: 23680625 Free PMC article. Review.
-
Actin depletion initiates events leading to granule secretion at the immunological synapse.Immunity. 2015 May 19;42(5):864-76. doi: 10.1016/j.immuni.2015.04.013. Immunity. 2015. PMID: 25992860 Free PMC article.
-
Actomyosin dynamics modulate microtubule deformation and growth during T-cell activation.Mol Biol Cell. 2021 Aug 19;32(18):1641-1653. doi: 10.1091/mbc.E20-10-0685. Epub 2021 Apr 7. Mol Biol Cell. 2021. PMID: 33826369 Free PMC article.
References
-
- Bubb M.R., Senderowicz A.M., Sausville E.A., Duncan K.L., Korn E.D. 1994. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 269:14869–14871 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

