Hepatitis C virus sensitizes host cells to TRAIL-induced apoptosis by up-regulating DR4 and DR5 via a MEK1-dependent pathway
- PMID: 22662193
- PMCID: PMC3360765
- DOI: 10.1371/journal.pone.0037700
Hepatitis C virus sensitizes host cells to TRAIL-induced apoptosis by up-regulating DR4 and DR5 via a MEK1-dependent pathway
Abstract
Background: Hepatitis C virus (HCV) is the leading cause of liver fibrosis, cirrhosis and hepatocellular carcinoma. It is believed that continuous liver cell apoptosis contributes to HCV pathogenesis. Recent studies have shown that HCV infection can sensitize host cells to TNF-related apoptosis-inducing ligand (TRAIL) induced apoptosis, but the mechanism by which HCV regulates the TRAIL pathway remains unclear.
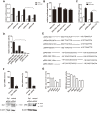
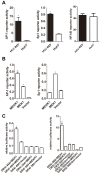
Methods and results: Using a sub-genomic replicon and full length virus, JFH-1, we demonstrate that HCV can sensitize host cells to TRAIL-induced apoptosis by up-regulating two TRAIL receptors, death receptor 4 (DR4) and death receptor 5 (DR5). Furthermore, the HCV replicon enhanced transcription of DR5 via Sp1, and the HCV-mediated up-regulation of DR4 and DR5 required MEK1 activity. HCV infection also stimulated the activity of MEK1, and the inhibition of MEK1 activity or the knockdown of MEK1 increased the replication of HCV.
Conclusions: Our studies demonstrate that HCV replication sensitizes host cells to TRAIL-induced apoptosis by up-regulating DR4 and DR5 via a MEK1 dependent pathway. These findings may help to further understand the pathogenesis of HCV infection and provide a therapeutic target.
Conflict of interest statement
Figures


Similar articles
-
TRAIL enhances apoptosis of human hepatocellular carcinoma cells sensitized by hepatitis C virus infection: therapeutic implications.PLoS One. 2014 Jun 13;9(6):e98171. doi: 10.1371/journal.pone.0098171. eCollection 2014. PLoS One. 2014. PMID: 24927176 Free PMC article.
-
Butein sensitizes human hepatoma cells to TRAIL-induced apoptosis via extracellular signal-regulated kinase/Sp1-dependent DR5 upregulation and NF-kappaB inactivation.Mol Cancer Ther. 2010 Jun;9(6):1583-95. doi: 10.1158/1535-7163.MCT-09-0942. Epub 2010 Jun 1. Mol Cancer Ther. 2010. PMID: 20515942
-
Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 up-regulation and proteasome-mediated c-FLIPS down-regulation.J Cell Biochem. 2008 Dec 15;105(6):1386-98. doi: 10.1002/jcb.21958. J Cell Biochem. 2008. PMID: 18980244
-
Regulation of the human TRAIL gene.Cancer Biol Ther. 2012 Oct;13(12):1143-51. doi: 10.4161/cbt.21354. Epub 2012 Aug 15. Cancer Biol Ther. 2012. PMID: 22892844 Free PMC article. Review.
-
Developing TRAIL/TRAIL death receptor-based cancer therapies.Cancer Metastasis Rev. 2018 Dec;37(4):733-748. doi: 10.1007/s10555-018-9728-y. Cancer Metastasis Rev. 2018. PMID: 29541897 Free PMC article. Review.
Cited by
-
Soluble TRAIL levels decreased in chronic hepatitis C treatment with pegylated interferon α plus ribavirin: association with viral responses.Int J Clin Exp Med. 2014 Dec 15;7(12):5650-6. eCollection 2014. Int J Clin Exp Med. 2014. PMID: 25664085 Free PMC article.
-
The role of anesthetic drugs in liver apoptosis.Hepat Mon. 2013 Aug 25;13(8):e13162. doi: 10.5812/hepatmon.13162. Hepat Mon. 2013. PMID: 24069040 Free PMC article. Review.
-
TRAIL enhances apoptosis of human hepatocellular carcinoma cells sensitized by hepatitis C virus infection: therapeutic implications.PLoS One. 2014 Jun 13;9(6):e98171. doi: 10.1371/journal.pone.0098171. eCollection 2014. PLoS One. 2014. PMID: 24927176 Free PMC article.
-
HTNV Sensitizes Host Toward TRAIL-Mediated Apoptosis-A Pivotal Anti-hantaviral Role of TRAIL.Front Immunol. 2020 Jun 19;11:1072. doi: 10.3389/fimmu.2020.01072. eCollection 2020. Front Immunol. 2020. PMID: 32636833 Free PMC article.
-
Hepatocellular carcinoma: targeting of oncogenic signaling networks in TRAIL resistant cancer cells.Mol Biol Rep. 2014 Oct;41(10):6909-17. doi: 10.1007/s11033-014-3577-8. Epub 2014 Jul 19. Mol Biol Rep. 2014. PMID: 25037270 Review.
References
-
- Wiley SR Schooley K, Smolak PJ, Din WS, Huang CP, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. - PubMed
-
- Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. - PubMed
-
- Liang X, Liu Y, Zhang Q, Gao L, Han L, et al. Hepatitis B virus sensitizes hepatocytes to TRAIL-induced apoptosis through Bax. J Immunol. 2007;178:503–510. - PubMed
-
- Lan L, Gorke S, Rau SJ, Zeisel MB, Hildt E, et al. Hepatitis C virus infection sensitizes human hepatocytes to TRAIL-induced apoptosis in a caspase 9-dependent manner. J Immunol. 2008;181:4926–4935. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

