Comparison of KRAS Mutation Assessment in Tumor DNA and Circulating Free DNA in Plasma and Serum Samples
- PMID: 22661904
- PMCID: PMC3362326
- DOI: 10.4137/CPath.S8798
Comparison of KRAS Mutation Assessment in Tumor DNA and Circulating Free DNA in Plasma and Serum Samples
Abstract
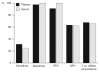
Testing for mutations in the KRAS oncogene for patients with metastatic colorectal cancer (mCRC) is generally performed using DNA from formalin-fixed paraffin-embedded tumor tissue; however, access to specimens can be limited and analysis challenging. This study assessed the identification of KRAS mutations in circulating free DNA (cfDNA) using a commercially available KRAS polymerase chain reaction (PCR) kit. Matched plasma, serum and tumor samples were available from 71 patients with mCRC who had received prior therapy but whose disease progressed following therapy. Yields of cfDNA from plasma and serum samples were comparable. Analyses were successful in 70/71 plasma-extracted samples (specificity: 97%, sensitivity: 31%) and 67/71 serum- extracted samples (specificity: 100%, sensitivity: 25%). This study demonstrates that KRAS mutations can be detected in cfDNA using a commercially available KRAS PCR kit, confirming cfDNA as a potential alternative source of tumor DNA in a diagnostic setting if access to archival tumor specimens is limited.
Keywords: KRAS; cfDNA; colorectal cancer; mutation.
Figures

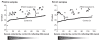
Similar articles
-
Comparison of KRAS mutation analysis of primary tumors and matched circulating cell-free DNA in plasmas of patients with colorectal cancer.Clin Chim Acta. 2014 Jun 10;433:284-9. doi: 10.1016/j.cca.2014.03.024. Epub 2014 Mar 29. Clin Chim Acta. 2014. PMID: 24685572
-
Clinical utility of KRAS status in circulating plasma DNA compared to archival tumour tissue from patients with metastatic colorectal cancer treated with anti-epidermal growth factor receptor therapy.Eur J Cancer. 2015 Nov;51(17):2678-85. doi: 10.1016/j.ejca.2015.06.118. Epub 2015 Oct 24. Eur J Cancer. 2015. PMID: 26508156 Clinical Trial.
-
Chemotherapy-associated clonal hematopoiesis mutations should be taken seriously in plasma cell-free DNA KRAS/NRAS/BRAF genotyping for metastatic colorectal cancer.Clin Biochem. 2021 Jun;92:46-53. doi: 10.1016/j.clinbiochem.2021.03.005. Epub 2021 Mar 15. Clin Biochem. 2021. PMID: 33737000
-
[Clinical implications of the concentration and EGFR/KRAS mutations of plasma cell free DNA of patients with lung cancer and esophageal cancer].Zhonghua Yi Xue Za Zhi. 2015 Dec 15;95(47):3839-42. Zhonghua Yi Xue Za Zhi. 2015. PMID: 27337801 Chinese.
-
Cell-Free DNA in Metastatic Colorectal Cancer: A Systematic Review and Meta-Analysis.Oncologist. 2017 Sep;22(9):1049-1055. doi: 10.1634/theoncologist.2016-0178. Epub 2017 Aug 4. Oncologist. 2017. PMID: 28778958 Free PMC article. Review.
Cited by
-
The diagnostic accuracy of circulating free DNA for the detection of KRAS mutation status in colorectal cancer: A meta-analysis.Cancer Med. 2019 Mar;8(3):1218-1231. doi: 10.1002/cam4.1989. Epub 2019 Feb 21. Cancer Med. 2019. PMID: 30791218 Free PMC article.
-
MutScan: fast detection and visualization of target mutations by scanning FASTQ data.BMC Bioinformatics. 2018 Jan 22;19(1):16. doi: 10.1186/s12859-018-2024-6. BMC Bioinformatics. 2018. PMID: 29357822 Free PMC article.
-
Liquid biopsy: monitoring cancer-genetics in the blood.Nat Rev Clin Oncol. 2013 Aug;10(8):472-84. doi: 10.1038/nrclinonc.2013.110. Epub 2013 Jul 9. Nat Rev Clin Oncol. 2013. PMID: 23836314 Review.
-
Next-generation sequencing technologies: breaking the sound barrier of human genetics.Mutagenesis. 2014 Sep;29(5):303-10. doi: 10.1093/mutage/geu031. Mutagenesis. 2014. PMID: 25150023 Free PMC article. Review.
-
Factors that influence quality and yield of circulating-free DNA: A systematic review of the methodology literature.Heliyon. 2018 Jul 25;4(7):e00699. doi: 10.1016/j.heliyon.2018.e00699. eCollection 2018 Jul. Heliyon. 2018. PMID: 30094369 Free PMC article.
References
-
- Markman B, Javier Ramos F, Capdevila J, Tabernero J. EGFR and KRAS in colorectal cancer. Adv Clin Chem. 2010;51:71–119. - PubMed
-
- Plesec TP, Hunt JL. KRAS mutation testing in colorectal cancer. Adv Anat Pathol. 2009;16:196–203. - PubMed
-
- van Krieken JH, Jung A, Kirchner T, et al. KRAS mutation testing for predicting response to anti-EGFR therapy for colorectal carcinoma: proposal for an European quality assurance program. Virchows Arch. 2008;453:417–31. - PubMed
-
- De Roock W, Piessevaux H, De Schutter J, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–15. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

