Mononuclear phagocyte intercellular crosstalk facilitates transmission of cell-targeted nanoformulated antiretroviral drugs to human brain endothelial cells
- PMID: 22661891
- PMCID: PMC3357981
- DOI: 10.2147/IJN.S29454
Mononuclear phagocyte intercellular crosstalk facilitates transmission of cell-targeted nanoformulated antiretroviral drugs to human brain endothelial cells
Abstract
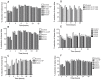
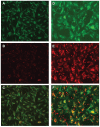
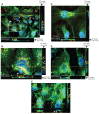
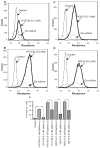
Despite the successes of antiretroviral therapy (ART), HIV-associated neurocognitive disorders remain prevalent in infected people. This is due, in part, to incomplete ART penetration across the blood-brain barrier (BBB) and lymph nodes and to the establishment of viral sanctuaries within the central nervous system. In efforts to improve ART delivery, our laboratories developed a macrophage-carriage system for nanoformulated crystalline ART (nanoART) (atazanavir, ritonavir, indinavir, and efavirenz). We demonstrate that nanoART transfer from mononuclear phagocytes (MP) to human brain microvascular endothelial cells (HBMEC) can be realized through cell-to-cell contacts, which can facilitate drug passage across the BBB. Coculturing of donor MP containing nanoART with recipient HBMEC facilitates intercellular particle transfer. NanoART uptake was observed in up to 52% of HBMEC with limited cytotoxicity. Folate coating of nanoART increased MP to HBMEC particle transfer by up to 77%. To translate the cell assays into relevant animal models of disease, ritonavir and atazanavir nanoformulations were injected into HIV-1-infected NOD/scid-γ(c)(null) mice reconstituted with human peripheral blood lymphocytes. Atazanavir and ritonavir levels in brains of mice treated with folate-coated nanoART were three- to four-fold higher than in mice treated with noncoated particles. This was associated with decreased viral load in the spleen and brain, and diminished brain CD11b-associated glial activation. We postulate that monocyte-macrophage transfer of nanoART to brain endothelial cells could facilitate drug entry into the brain.
Keywords: antiretroviral therapy; blood-brain barrier; folate; monocyte-endothelial cell interactions; nanoART; nanomedicine.
Figures
Similar articles
-
Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1-infected humanized mice.AIDS. 2012 Nov 13;26(17):2135-44. doi: 10.1097/QAD.0b013e328357f5ad. AIDS. 2012. PMID: 22824628 Free PMC article.
-
Pharmacodynamics of long-acting folic acid-receptor targeted ritonavir-boosted atazanavir nanoformulations.Biomaterials. 2015 Feb;41:141-50. doi: 10.1016/j.biomaterials.2014.11.012. Epub 2014 Dec 9. Biomaterials. 2015. PMID: 25522973 Free PMC article.
-
Macrophage folate receptor-targeted antiretroviral therapy facilitates drug entry, retention, antiretroviral activities and biodistribution for reduction of human immunodeficiency virus infections.Nanomedicine. 2013 Nov;9(8):1263-73. doi: 10.1016/j.nano.2013.05.003. Epub 2013 May 13. Nanomedicine. 2013. PMID: 23680933 Free PMC article.
-
Atazanavir/ritonavir: a review of its use in HIV therapy.Drugs Today (Barc). 2008 Feb;44(2):103-32. doi: 10.1358/dot.2008.44.2.1137107. Drugs Today (Barc). 2008. PMID: 18389089 Review.
-
Nano-ART and NeuroAIDS.Drug Deliv Transl Res. 2016 Oct;6(5):452-72. doi: 10.1007/s13346-016-0293-z. Drug Deliv Transl Res. 2016. PMID: 27137528 Review.
Cited by
-
Hydrophobic-core PEGylated graft copolymer-stabilized nanoparticles composed of insoluble non-nucleoside reverse transcriptase inhibitors exhibit strong anti-HIV activity.Nanomedicine. 2016 Nov;12(8):2405-2413. doi: 10.1016/j.nano.2016.07.004. Epub 2016 Jul 25. Nanomedicine. 2016. PMID: 27456163 Free PMC article.
-
Nose to brain delivery of antiretroviral drugs in the treatment of neuroAIDS.Mol Biomed. 2020;1(1):15. doi: 10.1186/s43556-020-00019-8. Epub 2020 Dec 10. Mol Biomed. 2020. PMID: 34765998 Free PMC article. Review.
-
Preclinical pharmacokinetics and tissue distribution of long-acting nanoformulated antiretroviral therapy.Antimicrob Agents Chemother. 2013 Jul;57(7):3110-20. doi: 10.1128/AAC.00267-13. Epub 2013 Apr 22. Antimicrob Agents Chemother. 2013. PMID: 23612193 Free PMC article.
-
Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1-infected humanized mice.AIDS. 2012 Nov 13;26(17):2135-44. doi: 10.1097/QAD.0b013e328357f5ad. AIDS. 2012. PMID: 22824628 Free PMC article.
-
Enabling nanomaterial, nanofabrication and cellular technologies for nanoneuromedicines.Nanomedicine. 2015 Apr;11(3):715-29. doi: 10.1016/j.nano.2014.12.013. Epub 2015 Jan 31. Nanomedicine. 2015. PMID: 25652894 Free PMC article. Review.
References
-
- Grant I, Sacktor N, McArthur J. HIV neurocognitive disorders. In: Gendelman HE, Grant I, Everall IP, Lipton SA, Swindells S, editors. The Neurology of AIDS. 2 ed. New York: Oxford University Press; 2005. pp. 357–373.
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS036126/NS/NINDS NIH HHS/United States
- P01 DA028555/DA/NIDA NIH HHS/United States
- P30 MH062261/MH/NIMH NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
- 2R01 NS034239/NS/NINDS NIH HHS/United States
- R01 MH081780/MH/NIMH NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R37 NS036126/NS/NINDS NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- 1P01DA028555-01/DA/NIDA NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
- P01 NS31492/NS/NINDS NIH HHS/United States
- R01MH081780/MH/NIMH NIH HHS/United States
- P01 MH64570/MH/NIMH NIH HHS/United States
- P20 RR15635/RR/NCRR NIH HHS/United States
- P20 RR015635/RR/NCRR NIH HHS/United States
- 2R37 NS36126/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

