Safety and activity of anti-PD-L1 antibody in patients with advanced cancer
- PMID: 22658128
- PMCID: PMC3563263
- DOI: 10.1056/NEJMoa1200694
Safety and activity of anti-PD-L1 antibody in patients with advanced cancer
Abstract
Background: Programmed death 1 (PD-1) protein, a T-cell coinhibitory receptor, and one of its ligands, PD-L1, play a pivotal role in the ability of tumor cells to evade the host's immune system. Blockade of interactions between PD-1 and PD-L1 enhances immune function in vitro and mediates antitumor activity in preclinical models.
Methods: In this multicenter phase 1 trial, we administered intravenous anti-PD-L1 antibody (at escalating doses ranging from 0.3 to 10 mg per kilogram of body weight) to patients with selected advanced cancers. Anti-PD-L1 antibody was administered every 14 days in 6-week cycles for up to 16 cycles or until the patient had a complete response or confirmed disease progression.
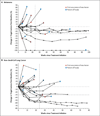
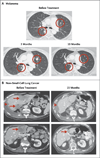
Results: As of February 24, 2012, a total of 207 patients--75 with non-small-cell lung cancer, 55 with melanoma, 18 with colorectal cancer, 17 with renal-cell cancer, 17 with ovarian cancer, 14 with pancreatic cancer, 7 with gastric cancer, and 4 with breast cancer--had received anti-PD-L1 antibody. The median duration of therapy was 12 weeks (range, 2 to 111). Grade 3 or 4 toxic effects that investigators considered to be related to treatment occurred in 9% of patients. Among patients with a response that could be evaluated, an objective response (a complete or partial response) was observed in 9 of 52 patients with melanoma, 2 of 17 with renal-cell cancer, 5 of 49 with non-small-cell lung cancer, and 1 of 17 with ovarian cancer. Responses lasted for 1 year or more in 8 of 16 patients with at least 1 year of follow-up.
Conclusions: Antibody-mediated blockade of PD-L1 induced durable tumor regression (objective response rate of 6 to 17%) and prolonged stabilization of disease (rates of 12 to 41% at 24 weeks) in patients with advanced cancers, including non-small-cell lung cancer, melanoma, and renal-cell cancer. (Funded by Bristol-Myers Squibb and others; ClinicalTrials.gov number, NCT00729664.).
Figures
Comment in
-
Tumor immunotherapy directed at PD-1.N Engl J Med. 2012 Jun 28;366(26):2517-9. doi: 10.1056/NEJMe1205943. Epub 2012 Jun 2. N Engl J Med. 2012. PMID: 22658126 No abstract available.
-
From ASCO-immunotherapy: programming cancer cell death.Nat Rev Clin Oncol. 2012 Jun 19;9(8):427. doi: 10.1038/nrclinonc.2012.104. Nat Rev Clin Oncol. 2012. PMID: 22710340 No abstract available.
-
The future of cancer treatment: will it include immunotherapy?Cancer Cell. 2012 Jul 10;22(1):7-8. doi: 10.1016/j.ccr.2012.06.009. Cancer Cell. 2012. PMID: 22789534
-
Re: Safety and activity of anti-PD-L1 antibody in patients with advanced cancer.J Urol. 2012 Dec;188(6):2148-9. doi: 10.1016/j.juro.2012.08.169. Epub 2012 Oct 18. J Urol. 2012. PMID: 23141220 No abstract available.
Similar articles
-
Safety, activity, and immune correlates of anti-PD-1 antibody in cancer.N Engl J Med. 2012 Jun 28;366(26):2443-54. doi: 10.1056/NEJMoa1200690. Epub 2012 Jun 2. N Engl J Med. 2012. PMID: 22658127 Free PMC article. Clinical Trial.
-
Nivolumab plus ipilimumab in advanced melanoma.N Engl J Med. 2013 Jul 11;369(2):122-33. doi: 10.1056/NEJMoa1302369. Epub 2013 Jun 2. N Engl J Med. 2013. PMID: 23724867 Free PMC article. Clinical Trial.
-
Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial.Lancet Oncol. 2015 Mar;16(3):257-65. doi: 10.1016/S1470-2045(15)70054-9. Epub 2015 Feb 20. Lancet Oncol. 2015. PMID: 25704439 Free PMC article. Clinical Trial.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
PD-1 Pathway Inhibitors: Immuno-Oncology Agents for Restoring Antitumor Immune Responses.Pharmacotherapy. 2016 Mar;36(3):317-34. doi: 10.1002/phar.1714. Pharmacotherapy. 2016. PMID: 26822752 Free PMC article. Review.
Cited by
-
Unlocking Benzosampangine's Potential: A Computational Approach to Investigating, Its Role as a PD-L1 Inhibitor in Tumor Immune Evasion via Molecular Docking, Dynamic Simulation, and ADMET Profiling.Bioinform Biol Insights. 2024 Nov 18;18:11779322241298591. doi: 10.1177/11779322241298591. eCollection 2024. Bioinform Biol Insights. 2024. PMID: 39564188 Free PMC article.
-
Landscape of targeted therapies for lung squamous cell carcinoma.Front Oncol. 2024 Oct 31;14:1467898. doi: 10.3389/fonc.2024.1467898. eCollection 2024. Front Oncol. 2024. PMID: 39544292 Free PMC article. Review.
-
In situ editing of tumour cell membranes induces aggregation and capture of PD-L1 membrane proteins for enhanced cancer immunotherapy.Nat Commun. 2024 Nov 9;15(1):9723. doi: 10.1038/s41467-024-54081-9. Nat Commun. 2024. PMID: 39521768 Free PMC article.
-
A PD-L1-Targeted Probe Cy5.5-A11 for In Vivo Imaging of Multiple Tumors.ACS Omega. 2024 Oct 17;9(43):43826-43833. doi: 10.1021/acsomega.4c06761. eCollection 2024 Oct 29. ACS Omega. 2024. PMID: 39494025 Free PMC article.
-
Tumour-intrinsic PDL1 signals regulate the Chk2 DNA damage response in cancer cells and mediate resistance to Chk1 inhibitors.Mol Cancer. 2024 Oct 30;23(1):242. doi: 10.1186/s12943-024-02147-z. Mol Cancer. 2024. PMID: 39478560 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
