Molecular basis of signaling specificity of insulin and IGF receptors: neglected corners and recent advances
- PMID: 22649417
- PMCID: PMC3355962
- DOI: 10.3389/fendo.2012.00034
Molecular basis of signaling specificity of insulin and IGF receptors: neglected corners and recent advances
Abstract
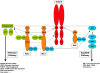
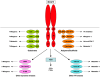
Insulin and insulin-like growth factor (IGF) receptors utilize common phosphoinositide 3-kinase/Akt and Ras/extracellular signal-regulated kinase signaling pathways to mediate a broad spectrum of "metabolic" and "mitogenic" responses. Specificity of insulin and IGF action in vivo must in part reflect expression of receptors and responsive pathways in different tissues but it is widely assumed that it is also determined by the ligand binding and signaling mechanisms of the receptors. This review focuses on receptor-proximal events in insulin/IGF signaling and examines their contribution to specificity of downstream responses. Insulin and IGF receptors may differ subtly in the efficiency with which they recruit their major substrates (IRS-1 and IRS-2 and Shc) and this could influence effectiveness of signaling to "metabolic" and "mitogenic" responses. Other substrates (Grb2-associated binder, downstream of kinases, SH2Bs, Crk), scaffolds (RACK1, β-arrestins, cytohesins), and pathways (non-receptor tyrosine kinases, phosphoinositide kinases, reactive oxygen species) have been less widely studied. Some of these components appear to be specifically involved in "metabolic" or "mitogenic" signaling but it has not been shown that this reflects receptor-preferential interaction. Very few receptor-specific interactions have been characterized, and their roles in signaling are unclear. Signaling specificity might also be imparted by differences in intracellular trafficking or feedback regulation of receptors, but few studies have directly addressed this possibility. Although published data are not wholly conclusive, no evidence has yet emerged for signaling mechanisms that are specifically engaged by insulin receptors but not IGF receptors or vice versa, and there is only limited evidence for differential activation of signaling mechanisms that are common to both receptors. Cellular context, rather than intrinsic receptor activity, therefore appears to be the major determinant of whether responses to insulin and IGFs are perceived as "metabolic" or "mitogenic."
Keywords: IGF receptor; adaptor; insulin receptor; kinase; scaffold; signaling; specificity; substrate.
Figures
Similar articles
-
Comparison of the insulin and insulin-like growth factor 1 mitogenic intracellular signaling pathways.Endocrinology. 1996 Oct;137(10):4427-34. doi: 10.1210/endo.137.10.8828504. Endocrinology. 1996. PMID: 8828504
-
Insulin receptor substrate 2 and Shc play different roles in insulin-like growth factor I signaling.J Biol Chem. 1998 Dec 18;273(51):34543-50. doi: 10.1074/jbc.273.51.34543. J Biol Chem. 1998. PMID: 9852124
-
Involvement of Raf-1 kinase and protein kinase C zeta in insulin-like growth factor I-induced brown adipocyte mitogenic signaling cascades: inhibition by cyclic adenosine 3',5'-monophosphate.Endocrinology. 1996 Sep;137(9):3832-41. doi: 10.1210/endo.137.9.8756554. Endocrinology. 1996. PMID: 8756554
-
Differential regulation of signaling pathways for insulin and insulin-like growth factor I.Acta Biochim Pol. 1999;46(1):51-60. Acta Biochim Pol. 1999. PMID: 10453981 Review.
-
Signaling via the insulin-like growth factor-I receptor: does it differ from insulin receptor signaling?Cytokine Growth Factor Rev. 1996 Aug;7(2):153-9. doi: 10.1016/1359-6101(96)00015-9. Cytokine Growth Factor Rev. 1996. PMID: 8899293 Review.
Cited by
-
IGF-1R/β-catenin signaling axis is involved in type 2 diabetic osteoporosis.J Zhejiang Univ Sci B. 2019 Oct.;20(10):838-848. doi: 10.1631/jzus.B1800648. J Zhejiang Univ Sci B. 2019. PMID: 31489803 Free PMC article.
-
A versatile insulin analog with high potency for both insulin and insulin-like growth factor 1 receptors: Structural implications for receptor binding.J Biol Chem. 2018 Oct 26;293(43):16818-16829. doi: 10.1074/jbc.RA118.004852. Epub 2018 Sep 13. J Biol Chem. 2018. PMID: 30213860 Free PMC article.
-
Agonist-Biased Signaling via Matrix Metalloproteinase-9 Promotes Extracellular Matrix Remodeling.Cells. 2018 Aug 26;7(9):117. doi: 10.3390/cells7090117. Cells. 2018. PMID: 30149671 Free PMC article. Review.
-
The host response to poly(lactide-co-glycolide) scaffolds protects mice from diet induced obesity and glucose intolerance.Biomaterials. 2019 Oct;217:119281. doi: 10.1016/j.biomaterials.2019.119281. Epub 2019 Jun 19. Biomaterials. 2019. PMID: 31260882 Free PMC article.
-
The Role of Insulin Receptor Isoforms in Diabetes and Its Metabolic and Vascular Complications.J Diabetes Res. 2017;2017:1403206. doi: 10.1155/2017/1403206. Epub 2017 Oct 19. J Diabetes Res. 2017. PMID: 29201918 Free PMC article. Review.
References
-
- Ahmed Z., Pillay T. S. (2003). Adapter protein with a pleckstrin homology (PH) and an Src homology 2 (SH2) domain (APS) and SH2-B enhance insulin-receptor autophosphorylation, extracellular-signal-regulated kinase and phosphoinositide 3-kinase-dependent signalling. Biochem. J. 371, 405–41210.1042/BJ20021589 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

