Peroxisome proliferator-activated receptor (PPAR)α and -γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way
- PMID: 22647601
- PMCID: PMC3386070
- DOI: 10.1073/pnas.1118458109
Peroxisome proliferator-activated receptor (PPAR)α and -γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way
Abstract
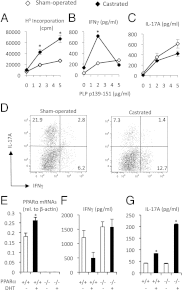
Women develop certain autoimmune diseases more often than men. It has been hypothesized that this may relate to the development of more robust T-helper (Th)1 responses in women. To test whether women exhibit a Th1 bias, we isolated naïve cluster of differentiation (CD)4(+) T cells from peripheral blood of healthy women and men and measured the proliferation and cytokine production by these cells in response to submaximal amounts of anti-CD3 and anti-CD28. We observed that CD4(+) T cells from women produced higher levels of IFNγ as well as tended to proliferate more than male CD4(+) T cells. Intriguingly, male CD4(+) T cells instead had a predilection toward IL-17A production. This sex dichotomy in Th cytokine production was found to be even more striking in the Swiss/Jackson Laboratory (SJL) mouse. Studies in mice and humans indicated that the sexual dimorphism in Th1 and Th17 cytokine production was dependent on the androgen status and the T-cell expression of peroxisome proliferator activated receptor (PPAR)α and PPARγ. Androgens increased PPARα and decreased PPARγ expression by human CD4(+) T cells. PPARα siRNA-mediated knockdown had the effect of increasing IFNγ by male CD4(+) T cells, while transfection of CD4(+) T cells with PPARγ siRNAs increased IL-17A production uniquely by female T cells. Together, our observations indicate that human T cells exhibit a sex difference in the production of IFNγ and IL-17A that may be driven by expressions of PPARα and PPARγ.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Peroxisome proliferator-activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity.J Exp Med. 2007 Feb 19;204(2):321-30. doi: 10.1084/jem.20061839. Epub 2007 Jan 29. J Exp Med. 2007. PMID: 17261635 Free PMC article.
-
Antagonizing Peroxisome Proliferator-Activated Receptor α Activity Selectively Enhances Th1 Immunity in Male Mice.J Immunol. 2015 Dec 1;195(11):5189-202. doi: 10.4049/jimmunol.1500449. Epub 2015 Oct 21. J Immunol. 2015. PMID: 26491197
-
PPAR activators as antiinflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation-associated arteriosclerosis.Circ Res. 2002 Apr 5;90(6):703-10. doi: 10.1161/01.res.0000014225.20727.8f. Circ Res. 2002. PMID: 11934839 Free PMC article.
-
Interleukin-23 promotes Th17 differentiation by inhibiting T-bet and FoxP3 and is required for elevation of interleukin-22, but not interleukin-21, in autoimmune experimental arthritis.Arthritis Rheum. 2010 Apr;62(4):1043-50. doi: 10.1002/art.27336. Arthritis Rheum. 2010. PMID: 20131264
-
Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms.Trends Immunol. 2007 Dec;28(12):551-8. doi: 10.1016/j.it.2007.09.003. Epub 2007 Nov 5. Trends Immunol. 2007. PMID: 17981503 Review.
Cited by
-
The influence of sex on neuroimmune communication, pain, and physiology.Biol Sex Differ. 2024 Oct 22;15(1):82. doi: 10.1186/s13293-024-00660-w. Biol Sex Differ. 2024. PMID: 39439003 Free PMC article. Review.
-
Independent effects of testosterone, estradiol, and sex chromosomes on gene expression in immune cells of trans- and cisgender individuals.bioRxiv [Preprint]. 2024 Oct 9:2024.10.08.617275. doi: 10.1101/2024.10.08.617275. bioRxiv. 2024. PMID: 39416170 Free PMC article. Preprint.
-
Stable and robust Xi and Y transcriptomes drive cell-type-specific autosomal and Xa responses in vivo and in vitro in four human cell types.Cell Genom. 2024 Sep 11;4(9):100628. doi: 10.1016/j.xgen.2024.100628. Epub 2024 Aug 6. Cell Genom. 2024. PMID: 39111319 Free PMC article.
-
Effective anti-tumor immune responses are orchestrated by immune cell partnership network that functions through tissue homeostatic pathways, not direct cytotoxicity.bioRxiv [Preprint]. 2024 Oct 10:2024.06.12.598563. doi: 10.1101/2024.06.12.598563. bioRxiv. 2024. PMID: 38903113 Free PMC article. Preprint.
-
The persistent inflammation in COPD: is autoimmunity the core mechanism?Eur Respir Rev. 2024 Mar 27;33(171):230137. doi: 10.1183/16000617.0137-2023. Print 2024 Jan 31. Eur Respir Rev. 2024. PMID: 38537947 Free PMC article. Review.
References
-
- Orton SM, et al. Canadian Collaborative Study Group Sex ratio of multiple sclerosis in Canada: A longitudinal study. Lancet Neurol. 2006;5:932–936. - PubMed
-
- Debouverie M, Pittion-Vouyovitch S, Louis S, Roederer T, Guillemin F. Increasing incidence of multiple sclerosis among women in Lorraine, Eastern France. Mult Scler. 2007;13:962–967. - PubMed
-
- Beeson PB. Age and sex associations of 40 autoimmune diseases. Am J Med. 1994;96:457–462. - PubMed
-
- Banwell B, Ghezzi A, Bar-Or A, Mikaeloff Y, Tardieu M. Multiple sclerosis in children: Clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurol. 2007;6:887–902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

