Pds5 promotes cohesin acetylation and stable cohesin-chromosome interaction
- PMID: 22640989
- PMCID: PMC3388792
- DOI: 10.1038/embor.2012.72
Pds5 promotes cohesin acetylation and stable cohesin-chromosome interaction
Abstract
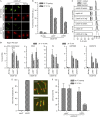
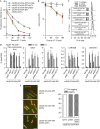
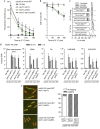
Pds5 and Wpl1 act as anti-establishment factors preventing sister-chromatid cohesion until counteracted in S-phase by the cohesin acetyl-transferase Eso1. However, Pds5 is also required to maintain sister-chromatid cohesion in G2. Here, we show that Pds5 is essential for cohesin acetylation by Eso1 and ensures the maintenance of cohesion by promoting a stable cohesin interaction with replicated chromosomes. The latter requires Eso1 only in the presence of Wapl, indicating that cohesin stabilization relies on Eso1 only to neutralize the anti-establishment activity. We suggest that Eso1 requires Pds5 to counteract anti-establishment. This allows both cohesion establishment and Pds5-dependent stable cohesin binding to chromosomes.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
DNA Topoisomerase II modulates acetyl-regulation of cohesin-mediated chromosome dynamics.Curr Genet. 2017 Oct;63(5):923-930. doi: 10.1007/s00294-017-0691-x. Epub 2017 Apr 5. Curr Genet. 2017. PMID: 28382430 Free PMC article.
-
Psm3 acetylation on conserved lysine residues is dispensable for viability in fission yeast but contributes to Eso1-mediated sister chromatid cohesion by antagonizing Wpl1.Mol Cell Biol. 2011 Apr;31(8):1771-86. doi: 10.1128/MCB.01284-10. Epub 2011 Feb 7. Mol Cell Biol. 2011. PMID: 21300781 Free PMC article.
-
Pds5 Regulates Sister-Chromatid Cohesion and Chromosome Bi-orientation through a Conserved Protein Interaction Module.Curr Biol. 2017 Apr 3;27(7):1005-1012. doi: 10.1016/j.cub.2017.02.066. Epub 2017 Mar 23. Curr Biol. 2017. PMID: 28343969
-
The expanding phenotypes of cohesinopathies: one ring to rule them all!Cell Cycle. 2019 Nov;18(21):2828-2848. doi: 10.1080/15384101.2019.1658476. Epub 2019 Sep 13. Cell Cycle. 2019. PMID: 31516082 Free PMC article. Review.
-
The Interplay of Cohesin and the Replisome at Processive and Stressed DNA Replication Forks.Cells. 2021 Dec 8;10(12):3455. doi: 10.3390/cells10123455. Cells. 2021. PMID: 34943967 Free PMC article. Review.
Cited by
-
Diverse developmental disorders from the one ring: distinct molecular pathways underlie the cohesinopathies.Front Genet. 2012 Sep 12;3:171. doi: 10.3389/fgene.2012.00171. eCollection 2012. Front Genet. 2012. PMID: 22988450 Free PMC article.
-
Functional interplay between cohesin and Smc5/6 complexes.Chromosoma. 2014 Oct;123(5):437-45. doi: 10.1007/s00412-014-0474-9. Epub 2014 Jul 1. Chromosoma. 2014. PMID: 24981336 Free PMC article. Review.
-
DNA Topoisomerase II modulates acetyl-regulation of cohesin-mediated chromosome dynamics.Curr Genet. 2017 Oct;63(5):923-930. doi: 10.1007/s00294-017-0691-x. Epub 2017 Apr 5. Curr Genet. 2017. PMID: 28382430 Free PMC article.
-
Crystal Structure of the Cohesin Gatekeeper Pds5 and in Complex with Kleisin Scc1.Cell Rep. 2016 Mar 8;14(9):2108-2115. doi: 10.1016/j.celrep.2016.02.020. Epub 2016 Feb 25. Cell Rep. 2016. PMID: 26923598 Free PMC article.
-
S. cerevisiae Cells Can Grow without the Pds5 Cohesin Subunit.mBio. 2022 Aug 30;13(4):e0142022. doi: 10.1128/mbio.01420-22. Epub 2022 Jun 16. mBio. 2022. PMID: 35708277 Free PMC article.
References
-
- Peters JM, Tedeschi A, Schmitz J (2008) The cohesin complex and its roles in chromosome biology. Genes Dev 22: 3089–3114 - PubMed
-
- Nasmyth K, Haering CH (2009) Cohesin: its roles and mechanisms. Annu Rev Genet 43: 525–558 - PubMed
-
- Nasmyth K (2011) Cohesin: a catenase with separate entry and exit gates? Nat Cell Biol 13: 1170–1177 - PubMed
-
- Onn I, Heidinger-Pauli JM, Guacci V, Unal E, Koshland DE (2008) Sister chromatid cohesion: a simple concept with a complex reality. Annu Rev Cell Dev Biol 24: 105–129 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

