TRIM16 acts as an E3 ubiquitin ligase and can heterodimerize with other TRIM family members
- PMID: 22629402
- PMCID: PMC3357404
- DOI: 10.1371/journal.pone.0037470
TRIM16 acts as an E3 ubiquitin ligase and can heterodimerize with other TRIM family members
Abstract
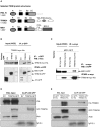
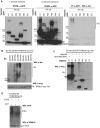
The TRIM family of proteins is distinguished by its tripartite motif (TRIM). Typically, TRIM proteins contain a RING finger domain, one or two B-box domains, a coiled-coil domain and the more variable C-terminal domains. TRIM16 does not have a RING domain but does harbour two B-box domains. Here we showed that TRIM16 homodimerized through its coiled-coil domain and heterodimerized with other TRIM family members; TRIM24, Promyelocytic leukaemia (PML) protein and Midline-1 (MID1). Although, TRIM16 has no classic RING domain, three-dimensional modelling of TRIM16 suggested that its B-box domains adopts RING-like folds leading to the hypothesis that TRIM16 acts as an ubiquitin ligase. Consistent with this hypothesis, we demonstrated that TRIM16, devoid of a classical RING domain had auto-polyubiquitination activity and acted as an E3 ubiquitin ligase in vivo and in vitro assays. Thus via its unique structure, TRIM16 possesses both heterodimerization function with other TRIM proteins and also has E3 ubiquitin ligase activity.
Conflict of interest statement
Figures



Similar articles
-
Detection and characterization of the in vitro e3 ligase activity of the human MID1 protein.J Mol Biol. 2011 Apr 8;407(4):505-20. doi: 10.1016/j.jmb.2011.01.048. Epub 2011 Feb 4. J Mol Biol. 2011. PMID: 21296087
-
Solution structure of the RBCC/TRIM B-box1 domain of human MID1: B-box with a RING.J Mol Biol. 2006 Apr 28;358(2):532-45. doi: 10.1016/j.jmb.2006.02.009. Epub 2006 Feb 20. J Mol Biol. 2006. PMID: 16529770
-
SUMO E3 ligase activity of TRIM proteins.Oncogene. 2011 Mar 3;30(9):1108-16. doi: 10.1038/onc.2010.462. Epub 2010 Oct 25. Oncogene. 2011. PMID: 20972456 Free PMC article.
-
Does it take two to tango? RING domain self-association and activity in TRIM E3 ubiquitin ligases.Biochem Soc Trans. 2020 Dec 18;48(6):2615-2624. doi: 10.1042/BST20200383. Biochem Soc Trans. 2020. PMID: 33170204 Free PMC article. Review.
-
TRIM proteins as RING finger E3 ubiquitin ligases.Adv Exp Med Biol. 2012;770:27-37. doi: 10.1007/978-1-4614-5398-7_3. Adv Exp Med Biol. 2012. PMID: 23630998 Review.
Cited by
-
Defense genes missing from the flight division.Dev Comp Immunol. 2013 Nov;41(3):377-88. doi: 10.1016/j.dci.2013.04.010. Epub 2013 Apr 24. Dev Comp Immunol. 2013. PMID: 23624185 Free PMC article. Review.
-
TRIM16 inhibits neuroblastoma cell proliferation through cell cycle regulation and dynamic nuclear localization.Cell Cycle. 2013 Mar 15;12(6):889-98. doi: 10.4161/cc.23825. Epub 2013 Feb 19. Cell Cycle. 2013. PMID: 23422002 Free PMC article.
-
E3 ubiquitin ligases: key regulators of osteogenesis and potential therapeutic targets for bone disorders.Front Cell Dev Biol. 2024 Aug 15;12:1447093. doi: 10.3389/fcell.2024.1447093. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39211390 Free PMC article. Review.
-
TRIM16 promotes aerobic glycolysis and pancreatic cancer metastasis by modulating the NIK-SIX1 axis in a ligase-independent manner.Am J Cancer Res. 2022 Nov 15;12(11):5205-5225. eCollection 2022. Am J Cancer Res. 2022. PMID: 36504902 Free PMC article.
-
Role of the E3 Ubiquitin Ligase TRIM4 in Predicting the Prognosis of Hepatocellular Carcinoma.J Cancer. 2020 Apr 6;11(14):4007-4014. doi: 10.7150/jca.37164. eCollection 2020. J Cancer. 2020. PMID: 32368282 Free PMC article.
References
-
- Joazeiro CA, Weissman AM. RING finger proteins: mediators of ubiquitin ligase activity. Cell. 2000;102:549–552. - PubMed
-
- Meroni G, Diez-Roux G, Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. Bioessays. 2005;27:1147–1157. - PubMed
-
- Cheung BB, Bell J, Raif A, Bohlken A, Yan J, et al. The estrogen-responsive B box protein is a novel regulator of the retinoid signal. J Biol Chem. 2006;281:18246–18256. - PubMed
-
- Raif A, Marshall GM, Bell JL, Koach J, Tan O, et al. The estrogen-responsive B box protein (EBBP) restores retinoid sensitivity in retinoid-resistant cancer cells via effects on histone acetylation. Cancer Lett. 2009;277:82–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

