Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia
- PMID: 22627678
- PMCID: PMC4118284
- DOI: 10.1038/leu.2012.115
Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia
Abstract
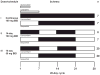
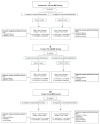
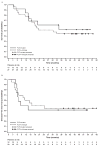
This phase 1b trial investigated several doses and schedules of midostaurin in combination with daunorubicin and cytarabine induction and high-dose cytarabine post-remission therapy in newly diagnosed patients with acute myeloid leukemia (AML). The discontinuation rate on the 50-mg twice-daily dose schedule was lower than 100 mg twice daily, and no grade 3/4 nausea or vomiting was seen. The complete remission rate for the midostaurin 50-mg twice-daily dose schedule was 80% (FMS-like tyrosine kinase 3 receptor (FLT3)-wild-type: 20 of 27 (74%), FLT3-mutant: 12 of 13 (92%)). Overall survival (OS) probabilities of patients with FLT3-mutant AML at 1 and 2 years (0.85 and 0.62, respectively) were similar to the FLT3-wild-type population (0.78 and 0.52, respectively). Midostaurin in combination with standard chemotherapy demonstrated high complete response and OS rates in newly diagnosed younger adults with AML, and was generally well tolerated at 50 mg twice daily for 14 days. A phase III prospective trial is ongoing (CALGB 10603, NCT00651261).
Conflict of interest statement
Figures

Similar articles
-
Midostaurin in Combination With Standard Chemotherapy for Treatment of Newly Diagnosed FMS-Like Tyrosine Kinase 3 (FLT3) Mutation-Positive Acute Myeloid Leukemia.Ann Pharmacother. 2018 Apr;52(4):364-369. doi: 10.1177/1060028017747900. Epub 2017 Dec 12. Ann Pharmacother. 2018. PMID: 29231051 Review.
-
Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation.N Engl J Med. 2017 Aug 3;377(5):454-464. doi: 10.1056/NEJMoa1614359. Epub 2017 Jun 23. N Engl J Med. 2017. PMID: 28644114 Free PMC article. Clinical Trial.
-
Midostaurin: A New Oral Agent Targeting FMS-Like Tyrosine Kinase 3-Mutant Acute Myeloid Leukemia.Pharmacotherapy. 2017 Dec;37(12):1586-1599. doi: 10.1002/phar.2039. Epub 2017 Nov 23. Pharmacotherapy. 2017. PMID: 28976600 Review.
-
Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3.J Clin Oncol. 2010 Oct 1;28(28):4339-45. doi: 10.1200/JCO.2010.28.9678. Epub 2010 Aug 23. J Clin Oncol. 2010. PMID: 20733134 Free PMC article. Clinical Trial.
-
Midostaurin/PKC412 for the treatment of newly diagnosed FLT3 mutation-positive acute myeloid leukemia.Expert Rev Hematol. 2017 Dec;10(12):1033-1045. doi: 10.1080/17474086.2017.1397510. Epub 2017 Oct 30. Expert Rev Hematol. 2017. PMID: 29069942 Review.
Cited by
-
Gilteritinib in the treatment of relapsed and refractory acute myeloid leukemia with a FLT3 mutation.Ther Adv Hematol. 2020 Jun 3;11:2040620720930614. doi: 10.1177/2040620720930614. eCollection 2020. Ther Adv Hematol. 2020. PMID: 32547718 Free PMC article. Review.
-
Recent drug approvals for acute myeloid leukemia.J Hematol Oncol. 2019 Sep 18;12(1):100. doi: 10.1186/s13045-019-0774-x. J Hematol Oncol. 2019. PMID: 31533852 Free PMC article. Review.
-
Use of FLT3 inhibitors in acute myeloid leukemia remission induction or salvage therapy: systematic review and meta-analysis.Cancer Manag Res. 2018 Aug 14;10:2635-2652. doi: 10.2147/CMAR.S166387. eCollection 2018. Cancer Manag Res. 2018. PMID: 30147364 Free PMC article.
-
New Targeted Agents in Acute Myeloid Leukemia: New Hope on the Rise.Int J Mol Sci. 2019 Apr 23;20(8):1983. doi: 10.3390/ijms20081983. Int J Mol Sci. 2019. PMID: 31018543 Free PMC article. Review.
-
A review of FLT3 inhibitors in acute myeloid leukemia.Blood Rev. 2022 Mar;52:100905. doi: 10.1016/j.blre.2021.100905. Epub 2021 Nov 3. Blood Rev. 2022. PMID: 34774343 Free PMC article. Review.
References
-
- Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. - PubMed
-
- Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001;61:7233–7239. - PubMed
-
- Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. - PubMed
-
- Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1:433–443. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

