Identification of two functionally distinct endosomal recycling pathways for dopamine D₂ receptor
- PMID: 22623662
- PMCID: PMC6622298
- DOI: 10.1523/JNEUROSCI.0008-12.2012
Identification of two functionally distinct endosomal recycling pathways for dopamine D₂ receptor
Abstract
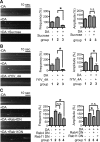
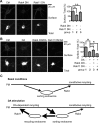
Dopamine D₂ receptor (DRD2) is important for normal function of the brain reward circuit. Lower DRD2 function in the brain increases the risk for substance abuse, obesity, attention deficit/hyperactivity disorder, and depression. Moreover, DRD2 is the target of most antipsychotics currently in use. It is well known that dopamine-induced DRD2 endocytosis is important for its desensitization. However, it remains controversial whether DRD2 is recycled back to the plasma membrane or targeted for degradation following dopamine stimulation. Here, we used total internal reflection fluorescent microscopy (TIRFM) to image DRD2 with a superecliptic pHluorin tagged to its N terminus. With these technical advances, we were able to directly visualize vesicular insertion events of DRD2 in cultured mouse striatal medium spiny neurons. We showed that insertion of DRD2 occurs on neuronal somatic and dendritic surfaces. Lateral diffusion of DRD2 was observed following its insertion. Most importantly, using our new approach, we uncovered two functionally distinct recycling pathways for DRD2: a constitutive recycling pathway and a dopamine activity-dependent recycling pathway. We further demonstrated that Rab4 plays an important role in constitutive DRD2 recycling, while Rab11 is required for dopamine activity-dependent DRD2 recycling. Finally, we demonstrated that the two DRD2 recycling pathways play distinct roles in determining DRD2 function: the Rab4-sensitive constitutive DRD2 recycling pathway determines steady-state surface expression levels of DRD2, whereas the Rab11-sensitive dopamine activity-dependent DRD2 recycling pathway is important for functional resensitization of DRD2. Our findings underscore the significance of endosomal recycling in regulation of DRD2 function.
Figures

Similar articles
-
Postendocytic sorting of constitutively internalized dopamine transporter in cell lines and dopaminergic neurons.J Biol Chem. 2010 Aug 27;285(35):27289-27301. doi: 10.1074/jbc.M110.131003. Epub 2010 Jun 15. J Biol Chem. 2010. PMID: 20551317 Free PMC article.
-
Differential Internalization Rates and Postendocytic Sorting of the Norepinephrine and Dopamine Transporters Are Controlled by Structural Elements in the N Termini.J Biol Chem. 2016 Mar 11;291(11):5634-5651. doi: 10.1074/jbc.M115.702050. Epub 2016 Jan 19. J Biol Chem. 2016. PMID: 26786096 Free PMC article.
-
Glucose transporter 3 is a rab11-dependent trafficking cargo and its transport to the cell surface is reduced in neurons of CAG140 Huntington's disease mice.Acta Neuropathol Commun. 2014 Dec 20;2:179. doi: 10.1186/s40478-014-0178-7. Acta Neuropathol Commun. 2014. PMID: 25526803 Free PMC article.
-
Polarized endocytic transport: the roles of Rab11 and Rab11-FIPs in regulating cell polarity.Histol Histopathol. 2009 Sep;24(9):1171-80. doi: 10.14670/HH-24.1171. Histol Histopathol. 2009. PMID: 19609864 Free PMC article. Review.
-
The Endosomal Recycling Pathway-At the Crossroads of the Cell.Int J Mol Sci. 2020 Aug 23;21(17):6074. doi: 10.3390/ijms21176074. Int J Mol Sci. 2020. PMID: 32842549 Free PMC article. Review.
Cited by
-
Tumor suppressor DRD2 facilitates M1 macrophages and restricts NF-κB signaling to trigger pyroptosis in breast cancer.Theranostics. 2021 Mar 5;11(11):5214-5231. doi: 10.7150/thno.58322. eCollection 2021. Theranostics. 2021. PMID: 33859743 Free PMC article.
-
Whole-transcriptome microarray analysis reveals regulation of Rab4 by RBM5 in neurons.Neuroscience. 2017 Oct 11;361:93-107. doi: 10.1016/j.neuroscience.2017.08.014. Epub 2017 Aug 15. Neuroscience. 2017. PMID: 28818525 Free PMC article.
-
Imaging GPCRs trafficking and signaling with total internal reflection fluorescence microscopy in cultured neurons.Methods Cell Biol. 2016;132:25-33. doi: 10.1016/bs.mcb.2015.10.002. Epub 2015 Dec 24. Methods Cell Biol. 2016. PMID: 26928537 Free PMC article.
-
Imaging pHluorin-tagged receptor insertion to the plasma membrane in primary cultured mouse neurons.J Vis Exp. 2012 Nov 20;(69):4450. doi: 10.3791/4450. J Vis Exp. 2012. PMID: 23208071 Free PMC article.
-
Effects of social defeat stress on dopamine D2 receptor isoforms and proteins involved in intracellular trafficking.Behav Brain Funct. 2018 Oct 8;14(1):16. doi: 10.1186/s12993-018-0148-5. Behav Brain Funct. 2018. PMID: 30296947 Free PMC article.
References
-
- Binda AV, Kabbani N, Lin R, Levenson R. D2 and D3 dopamine receptor cell surface localization mediated by interaction with protein 4.1N. Mol Pharmacol. 2002;62:507–513. - PubMed
-
- Bunzow JR, Van Tol HH, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988;336:783–787. - PubMed
-
- Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1:179–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
