Apolipoprotein E controls cerebrovascular integrity via cyclophilin A
- PMID: 22622580
- PMCID: PMC4047116
- DOI: 10.1038/nature11087
Apolipoprotein E controls cerebrovascular integrity via cyclophilin A
Erratum in
-
Author Correction: Apolipoprotein E controls cerebrovascular integrity via cyclophilin A.Nature. 2023 May;617(7961):E12. doi: 10.1038/s41586-023-06118-0. Nature. 2023. PMID: 37142785 No abstract available.
Abstract
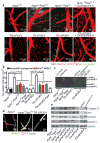
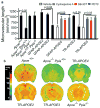
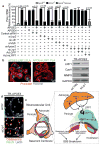
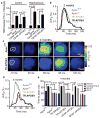
Human apolipoprotein E has three isoforms: APOE2, APOE3 and APOE4. APOE4 is a major genetic risk factor for Alzheimer's disease and is associated with Down's syndrome dementia and poor neurological outcome after traumatic brain injury and haemorrhage. Neurovascular dysfunction is present in normal APOE4 carriers and individuals with APOE4-associated disorders. In mice, lack of Apoe leads to blood-brain barrier (BBB) breakdown, whereas APOE4 increases BBB susceptibility to injury. How APOE genotype affects brain microcirculation remains elusive. Using different APOE transgenic mice, including mice with ablation and/or inhibition of cyclophilin A (CypA), here we show that expression of APOE4 and lack of murine Apoe, but not APOE2 and APOE3, leads to BBB breakdown by activating a proinflammatory CypA-nuclear factor-κB-matrix-metalloproteinase-9 pathway in pericytes. This, in turn, leads to neuronal uptake of multiple blood-derived neurotoxic proteins, and microvascular and cerebral blood flow reductions. We show that the vascular defects in Apoe-deficient and APOE4-expressing mice precede neuronal dysfunction and can initiate neurodegenerative changes. Astrocyte-secreted APOE3, but not APOE4, suppressed the CypA-nuclear factor-κB-matrix-metalloproteinase-9 pathway in pericytes through a lipoprotein receptor. Our data suggest that CypA is a key target for treating APOE4-mediated neurovascular injury and the resulting neuronal dysfunction and degeneration.
Figures

Comment in
-
Alzheimer's disease: A breach in the blood-brain barrier.Nature. 2012 May 23;485(7399):451-2. doi: 10.1038/485451a. Nature. 2012. PMID: 22622564 No abstract available.
Similar articles
-
Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease.JAMA Neurol. 2013 Apr;70(4):440-4. doi: 10.1001/jamaneurol.2013.2152. JAMA Neurol. 2013. PMID: 23400708 Free PMC article. Review.
-
Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer's disease.J Cereb Blood Flow Metab. 2016 Jan;36(1):216-27. doi: 10.1038/jcbfm.2015.44. J Cereb Blood Flow Metab. 2016. PMID: 25757756 Free PMC article.
-
Apolipoprotein E4 impairs spontaneous blood brain barrier repair following traumatic brain injury.Mol Neurodegener. 2018 Apr 4;13(1):17. doi: 10.1186/s13024-018-0249-5. Mol Neurodegener. 2018. PMID: 29618365 Free PMC article.
-
Differential Signaling Mediated by ApoE2, ApoE3, and ApoE4 in Human Neurons Parallels Alzheimer's Disease Risk.J Neurosci. 2019 Sep 11;39(37):7408-7427. doi: 10.1523/JNEUROSCI.2994-18.2019. Epub 2019 Jul 22. J Neurosci. 2019. PMID: 31331998 Free PMC article.
-
Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders.J Mol Med (Berl). 2016 Jul;94(7):739-46. doi: 10.1007/s00109-016-1427-y. Epub 2016 Jun 9. J Mol Med (Berl). 2016. PMID: 27277824 Free PMC article. Review.
Cited by
-
Investigating the Interplay between Cardiovascular and Neurodegenerative Disease.Biology (Basel). 2024 Sep 26;13(10):764. doi: 10.3390/biology13100764. Biology (Basel). 2024. PMID: 39452073 Free PMC article. Review.
-
Clearance systems in the brain-implications for Alzheimer disease.Nat Rev Neurol. 2015 Aug;11(8):457-70. doi: 10.1038/nrneurol.2015.119. Epub 2015 Jul 21. Nat Rev Neurol. 2015. PMID: 26195256 Free PMC article. Review.
-
Interaction of age and APOE genotype on cerebral blood flow at rest.J Alzheimers Dis. 2013;34(4):921-35. doi: 10.3233/JAD-121897. J Alzheimers Dis. 2013. PMID: 23302659 Free PMC article.
-
Reactive astrocytes transduce inflammation in a blood-brain barrier model through a TNF-STAT3 signaling axis and secretion of alpha 1-antichymotrypsin.Nat Commun. 2022 Nov 2;13(1):6581. doi: 10.1038/s41467-022-34412-4. Nat Commun. 2022. PMID: 36323693 Free PMC article.
-
Peripheral Blood Transcriptome in Patients with Sarcoidosis-Associated Uveitis.Ocul Immunol Inflamm. 2022 Jul;30(5):1074-1077. doi: 10.1080/09273948.2020.1861306. Epub 2021 Mar 4. Ocul Immunol Inflamm. 2022. PMID: 33661066 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

