Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models
- PMID: 22615798
- PMCID: PMC3352934
- DOI: 10.1371/journal.pone.0036713
Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models
Abstract
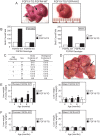
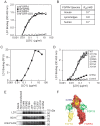
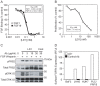
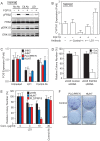
The fibroblast growth factor (FGF)-FGF receptor (FGFR) signaling system plays critical roles in a variety of normal developmental and physiological processes. It is also well documented that dysregulation of FGF-FGFR signaling may have important roles in tumor development and progression. The FGFR4-FGF19 signaling axis has been implicated in the development of hepatocellular carcinomas (HCCs) in mice, and potentially in humans. In this study, we demonstrate that FGFR4 is required for hepatocarcinogenesis; the progeny of FGF19 transgenic mice, which have previously been shown to develop HCCs, bred with FGFR4 knockout mice fail to develop liver tumors. To further test the importance of FGFR4 in HCC, we developed a blocking anti-FGFR4 monoclonal antibody (LD1). LD1 inhibited: 1) FGF1 and FGF19 binding to FGFR4, 2) FGFR4-mediated signaling, colony formation, and proliferation in vitro, and 3) tumor growth in a preclinical model of liver cancer in vivo. Finally, we show that FGFR4 expression is elevated in several types of cancer, including liver cancer, as compared to normal tissues. These findings suggest a modulatory role for FGFR4 in the development and progression of hepatocellular carcinoma and that FGFR4 may be an important and novel therapeutic target in treating this disease.
Conflict of interest statement
Figures
Similar articles
-
First Selective Small Molecule Inhibitor of FGFR4 for the Treatment of Hepatocellular Carcinomas with an Activated FGFR4 Signaling Pathway.Cancer Discov. 2015 Apr;5(4):424-37. doi: 10.1158/2159-8290.CD-14-1029. Epub 2015 Mar 16. Cancer Discov. 2015. PMID: 25776529
-
Fibroblast Growth Factor 19-Mediated Up-regulation of SYR-Related High-Mobility Group Box 18 Promotes Hepatocellular Carcinoma Metastasis by Transactivating Fibroblast Growth Factor Receptor 4 and Fms-Related Tyrosine Kinase 4.Hepatology. 2020 May;71(5):1712-1731. doi: 10.1002/hep.30951. Epub 2020 Feb 10. Hepatology. 2020. PMID: 31529503
-
Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma.BMC Cancer. 2012 Feb 6;12:56. doi: 10.1186/1471-2407-12-56. BMC Cancer. 2012. PMID: 22309595 Free PMC article.
-
FGF19-FGFR4 Signaling in Hepatocellular Carcinoma.Cells. 2019 Jun 4;8(6):536. doi: 10.3390/cells8060536. Cells. 2019. PMID: 31167419 Free PMC article. Review.
-
Fibroblast Growth Factor Receptor 4 (FGFR4) Selective Inhibitors as Hepatocellular Carcinoma Therapy: Advances and Prospects.J Med Chem. 2019 Mar 28;62(6):2905-2915. doi: 10.1021/acs.jmedchem.8b01531. Epub 2018 Nov 16. J Med Chem. 2019. PMID: 30403487 Review.
Cited by
-
Thrombocytosis and hepatocellular carcinoma.Dig Dis Sci. 2013 Jun;58(6):1790-6. doi: 10.1007/s10620-012-2527-3. Epub 2013 Jan 12. Dig Dis Sci. 2013. PMID: 23314854 Free PMC article.
-
Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances.F1000Res. 2016 May 12;5:F1000 Faculty Rev-879. doi: 10.12688/f1000research.6946.1. eCollection 2016. F1000Res. 2016. PMID: 27239288 Free PMC article. Review.
-
Isoform-specific inhibition of FGFR signaling achieved by a de-novo-designed mini-protein.Cell Rep. 2022 Oct 25;41(4):111545. doi: 10.1016/j.celrep.2022.111545. Cell Rep. 2022. PMID: 36288716 Free PMC article.
-
Deguelin-induced blockade of PI3K/protein kinase B/MAP kinase signaling in zebrafish and breast cancer cell lines is mediated by down-regulation of fibroblast growth factor receptor 4 activity.Pharmacol Res Perspect. 2016 Feb 23;4(2):e00212. doi: 10.1002/prp2.212. eCollection 2016 Apr. Pharmacol Res Perspect. 2016. PMID: 27069628 Free PMC article.
-
Novel combination of sorafenib and celecoxib provides synergistic anti-proliferative and pro-apoptotic effects in human liver cancer cells.PLoS One. 2013 Jun 12;8(6):e65569. doi: 10.1371/journal.pone.0065569. Print 2013. PLoS One. 2013. PMID: 23776502 Free PMC article.
References
-
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. - PubMed
-
- Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–197. - PubMed
-
- Bange J, Prechtl D, Cheburkin Y, Specht K, Harbeck N, et al. Cancer progression and tumor cell motility are associated with the FGFR4 Arg(388) allele. Cancer Res. 2002;62:840–847. - PubMed
-
- Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

