Phase II trial of vorinostat with idarubicin and cytarabine for patients with newly diagnosed acute myelogenous leukemia or myelodysplastic syndrome
- PMID: 22585696
- PMCID: PMC4879705
- DOI: 10.1200/JCO.2011.38.3265
Phase II trial of vorinostat with idarubicin and cytarabine for patients with newly diagnosed acute myelogenous leukemia or myelodysplastic syndrome
Abstract
Purpose: To evaluate the safety and efficacy of the combination of the histone deacetylase inhibitor vorinostat with idarubicin and ara-C (cytarabine) in patients with acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS).
Patients and methods: Patients with previously untreated AML or higher-risk MDS age 15 to 65 years with appropriate organ function and no core-binding factor abnormality were candidates. Induction therapy was vorinostat 500 mg orally three times a day (days 1 to 3), idarubin 12 mg/m(2) intravenously (IV) daily × 3 (days 4 to 6), and cytarabine 1.5 g/m(2) IV as a continuous infusion daily for 3 or 4 days (days 4 to 7). Patients in remission could be treated with five cycles of consolidation therapy and up to 12 months of maintenance therapy with single-agent vorinostat. The study was designed to stop early if either excess toxicity or low probability of median event-free survival (EFS) of more than 28 weeks was likely.
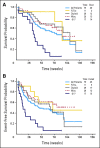
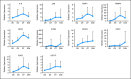
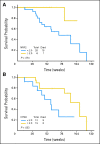
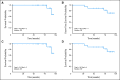
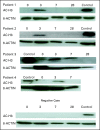
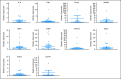
Results: After a three-patient run-in phase, 75 patients were treated. Median age was 52 years (range, 19 to 65 years), 29 patients (39%) were cytogenetically normal, and 11 (15%) had FLT-3 internal tandem duplication (ITD). No excess vorinostat-related toxicity was observed. Induction mortality was 4%. EFS was 47 weeks (range, 3 to 134 weeks), and overall survival was 82 weeks (range, 3 to 134 weeks). Overall response rate (ORR) was 85%, including 76% complete response (CR) and 9% in CR with incomplete platelet recovery. ORR was 93% in diploid patients and 100% in FLT-3 ITD patients. Levels of NRF2 and CYBB were associated with longer survival.
Conclusion: The combination of vorinostat with idarubicin and cytarabine is safe and active in AML.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Similar articles
-
Idarubicin, cytarabine, and topotecan in patients with refractory or relapsed acute myelogenous leukemia and high-risk myelodysplastic syndrome.Am J Hematol. 2001 Dec;68(4):237-45. doi: 10.1002/ajh.1188. Am J Hematol. 2001. PMID: 11754412
-
Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a single-arm, phase 2 study.Lancet Haematol. 2019 Sep;6(9):e480-e488. doi: 10.1016/S2352-3026(19)30114-0. Epub 2019 Aug 7. Lancet Haematol. 2019. PMID: 31400961 Free PMC article. Clinical Trial.
-
A phase 1-2 study of a farnesyltransferase inhibitor, tipifarnib, combined with idarubicin and cytarabine for patients with newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndrome.Cancer. 2011 Mar 15;117(6):1236-44. doi: 10.1002/cncr.25575. Epub 2010 Oct 19. Cancer. 2011. PMID: 20960519 Free PMC article. Clinical Trial.
-
Long-term follow-up of Cladribine, high-dose Cytarabine, and Idarubicin as salvage treatment for relapsed acute myeloid leukemia and literature review.Eur J Haematol. 2020 Jun;104(6):538-545. doi: 10.1111/ejh.13395. Epub 2020 Mar 10. Eur J Haematol. 2020. PMID: 32049382 Review.
-
Can we improve outcomes in patients with acute myelogenous leukemia? Incorporating HDAC inhibitors into front-line therapy.Best Pract Res Clin Haematol. 2012 Dec;25(4):427-35. doi: 10.1016/j.beha.2012.10.005. Epub 2012 Oct 25. Best Pract Res Clin Haematol. 2012. PMID: 23200539 Review.
Cited by
-
Identification of differentially expressed genes induced by aberrant methylation in acute myeloid leukemia using integrated bioinformatics analyses.Oncol Lett. 2022 Sep 13;24(5):383. doi: 10.3892/ol.2022.13503. eCollection 2022 Nov. Oncol Lett. 2022. PMID: 36238356 Free PMC article.
-
Overview of therapy and strategies for optimizing outcomes in de novo pediatric acute myeloid leukemia.Paediatr Drugs. 2014 Jun;16(3):213-27. doi: 10.1007/s40272-014-0067-3. Paediatr Drugs. 2014. PMID: 24639021 Review.
-
Strategies to modulate heritable epigenetic defects in cellular machinery: lessons from nature.Pharmaceuticals (Basel). 2012 Dec 27;6(1):1-24. doi: 10.3390/ph6010001. Pharmaceuticals (Basel). 2012. PMID: 24275784 Free PMC article.
-
Immunosuppressive Glycodelin A is an independent marker for poor prognosis in endometrial cancer.BMC Cancer. 2013 Dec 30;13:616. doi: 10.1186/1471-2407-13-616. BMC Cancer. 2013. PMID: 24377825 Free PMC article.
-
Epigenetic therapies in acute myeloid leukemia: the role of hypomethylating agents, histone deacetylase inhibitors and the combination of hypomethylating agents with histone deacetylase inhibitors.Chin Med J (Engl). 2020 Mar 20;133(6):699-715. doi: 10.1097/CM9.0000000000000685. Chin Med J (Engl). 2020. PMID: 32044818 Free PMC article. Review.
References
-
- Kantarjian HM, Erba HP, Claxton D, et al. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol. 2010;28:549–555. - PubMed
-
- Garcia-Manero G, Yang H, Bueso-Ramos C, et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008;111:1060–1066. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

