Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system
- PMID: 22581922
- PMCID: PMC3365576
- DOI: 10.1161/CIRCRESAHA.111.243972
Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system
Abstract
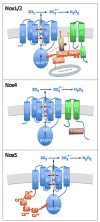
The NADPH oxidase (Nox) enzymes are critical mediators of cardiovascular physiology and pathophysiology. These proteins are expressed in virtually all cardiovascular cells, and regulate such diverse functions as differentiation, proliferation, apoptosis, senescence, inflammatory responses and oxygen sensing. They target a number of important signaling molecules, including kinases, phosphatases, transcription factors, ion channels, and proteins that regulate the cytoskeleton. Nox enzymes have been implicated in many different cardiovascular pathologies: atherosclerosis, hypertension, cardiac hypertrophy and remodeling, angiogenesis and collateral formation, stroke, and heart failure. In this review, we discuss in detail the biochemistry of Nox enzymes expressed in the cardiovascular system (Nox1, 2, 4, and 5), their roles in cardiovascular cell biology, and their contributions to disease development.
Figures
Similar articles
-
Inhibitors of Src Family Kinases, Inducible Nitric Oxide Synthase, and NADPH Oxidase as Potential CNS Drug Targets for Neurological Diseases.CNS Drugs. 2021 Jan;35(1):1-20. doi: 10.1007/s40263-020-00787-5. Epub 2021 Jan 30. CNS Drugs. 2021. PMID: 33515429 Free PMC article. Review.
-
Direct interaction between Tks proteins and the N-terminal proline-rich region (PRR) of NoxA1 mediates Nox1-dependent ROS generation.Eur J Cell Biol. 2011 Feb-Mar;90(2-3):164-71. doi: 10.1016/j.ejcb.2010.05.007. Epub 2010 Jul 6. Eur J Cell Biol. 2011. PMID: 20609497 Free PMC article.
-
Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Heart Disease and Diabetes.2023 Dec 20. In: Lawrence JM, Casagrande SS, Herman WH, Wexler DJ, Cefalu WT, editors. Diabetes in America [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); 2023–. 2023 Dec 20. In: Lawrence JM, Casagrande SS, Herman WH, Wexler DJ, Cefalu WT, editors. Diabetes in America [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); 2023–. PMID: 38117924 Free Books & Documents. Review.
Cited by
-
Reactive oxygen species in hypertension.Nat Rev Cardiol. 2025 Jan;22(1):20-37. doi: 10.1038/s41569-024-01062-6. Epub 2024 Jul 24. Nat Rev Cardiol. 2025. PMID: 39048744 Review.
-
Oxidative Stress and Hypertension.Circ Res. 2021 Apr 2;128(7):993-1020. doi: 10.1161/CIRCRESAHA.121.318063. Epub 2021 Apr 1. Circ Res. 2021. PMID: 33793335 Free PMC article. Review.
-
Nox2 contributes to the arterial endothelial specification of mouse induced pluripotent stem cells by upregulating Notch signaling.Sci Rep. 2016 Sep 19;6:33737. doi: 10.1038/srep33737. Sci Rep. 2016. PMID: 27642005 Free PMC article.
-
A small molecule inhibitor of Nox2 and Nox4 improves contractile function after ischemia-reperfusion in the mouse heart.Sci Rep. 2021 Jun 7;11(1):11970. doi: 10.1038/s41598-021-91575-8. Sci Rep. 2021. PMID: 34099836 Free PMC article.
-
Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase.J Mol Cell Cardiol. 2013 Sep;62:72-9. doi: 10.1016/j.yjmcc.2013.04.019. Epub 2013 May 2. J Mol Cell Cardiol. 2013. PMID: 23643589 Free PMC article. Review.
References
-
- Bedard K, Krause KH. The NOX family of ros-generating NADPH oxidases: Physiology and pathophysiology. Physiological reviews. 2007;87:245–313. - PubMed
-
- Bayraktutan U, Blayney L, Shah AM. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:1903–1911. - PubMed
-
- Hohler B, Holzapfel B, Kummer W. NADPH oxidase subunits and superoxide production in porcine pulmonary artery endothelial cells. Histochemistry and cell biology. 2000;114:29–37. - PubMed
-
- Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: Specific features, expression, and regulation. American journal of physiology. Regulatory, integrative and comparative physiology. 2003;285:R277–297. - PubMed
-
- Sumimoto H. Structure, regulation and evolution of nox-family NADPH oxidases that produce reactive oxygen species. The FEBS journal. 2008;275:3249–3277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

