Lipopolysaccharide-activated dendritic cells: "exhausted" or alert and waiting?
- PMID: 22561154
- PMCID: PMC3370068
- DOI: 10.4049/jimmunol.1102868
Lipopolysaccharide-activated dendritic cells: "exhausted" or alert and waiting?
Abstract
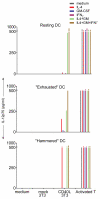
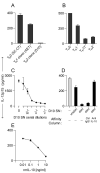
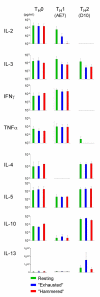
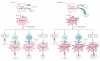
LPS-activated dendritic cells (DCs) are thought to follow a set program in which they secrete inflammatory cytokines (such as IL-12) and then become refractory to further stimulation (i.e., "exhausted"). In this study, we show that mouse DCs do indeed lose their responsiveness to LPS, but nevertheless remain perfectly capable of making inflammatory cytokines in response to signals from activated T cells and to CD40-ligand and soluble T cell-derived signals. Furthermore, far from being rigidly programmed by the original activating stimulus, the DCs retained sufficient plasticity to respond differentially to interactions with Th0, Th1, Th2, and Th17 T cells. These data suggest that LPS activation does not exhaust DCs but rather primes them for subsequent signals from T cells.
Figures


Similar articles
-
T-cell control of IL-12p75 production.Scand J Immunol. 2006 Aug;64(2):83-92. doi: 10.1111/j.1365-3083.2006.01767.x. Scand J Immunol. 2006. PMID: 16867152
-
Transglutaminase 2 on the surface of dendritic cells is proposed to be involved in dendritic cell-T cell interaction.Cell Immunol. 2014 May-Jun;289(1-2):55-62. doi: 10.1016/j.cellimm.2014.03.008. Epub 2014 Mar 31. Cell Immunol. 2014. PMID: 24727157
-
Lipopolysaccharide-induced suppression of airway Th2 responses does not require IL-12 production by dendritic cells.J Immunol. 2003 Oct 1;171(7):3645-54. doi: 10.4049/jimmunol.171.7.3645. J Immunol. 2003. PMID: 14500662
-
High dose lipopolysaccharide triggers polarization of mouse thymic Th17 cells in vitro in the presence of mature dendritic cells.Cell Immunol. 2012;274(1-2):98-108. doi: 10.1016/j.cellimm.2012.01.006. Epub 2012 Feb 6. Cell Immunol. 2012. PMID: 22361175
-
The dynamics of dendritic cell-T cell interactions in priming and tolerance.Curr Opin Immunol. 2006 Aug;18(4):491-5. doi: 10.1016/j.coi.2006.03.021. Epub 2006 Jun 12. Curr Opin Immunol. 2006. PMID: 16765575 Review.
Cited by
-
Annexin A5 increases survival in murine sepsis model by inhibiting HMGB1-mediated pro-inflammation and coagulation.Mol Med. 2016 Sep;22:424-436. doi: 10.2119/molmed.2016.00026. Epub 2016 Jul 6. Mol Med. 2016. PMID: 27447360 Free PMC article.
-
Making many from few: IL-12p40 as a model for the combinatorial assembly of heterodimeric cytokines.Cytokine. 2015 Nov;76(1):53-7. doi: 10.1016/j.cyto.2015.07.026. Epub 2015 Aug 1. Cytokine. 2015. PMID: 26242928 Free PMC article. Review.
-
Inhibition of EZH2 methyltransferase decreases immunoediting of mesothelioma cells by autologous macrophages through a PD-1-dependent mechanism.JCI Insight. 2019 Sep 19;4(18):e128474. doi: 10.1172/jci.insight.128474. JCI Insight. 2019. PMID: 31534051 Free PMC article.
-
Ex Vivo Endotoxin Stimulation of Blood for Predicting Survival in Patients With Sepsis: A Systematic Review.CHEST Crit Care. 2023 Dec;1(3):100029. doi: 10.1016/j.chstcc.2023.100029. Epub 2023 Oct 30. CHEST Crit Care. 2023. PMID: 38148988 Free PMC article.
-
Functional evaluation of dendritic cells and extracellular vesicles as immunotherapy for breast cancer.Oncogene. 2024 Jan;43(5):319-327. doi: 10.1038/s41388-023-02893-2. Epub 2023 Nov 29. Oncogene. 2024. PMID: 38030790 Free PMC article.
References
-
- Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. - PubMed
-
- Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. - PubMed
-
- Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, Ramwadhdoebe TH, Gorter A, Welters MJ, van Hall T, van der Burg SH. M2 Macrophages Induced by Prostaglandin E2 and IL-6 from Cervical Carcinoma Are Switched to Activated M1 Macrophages by CD4+ Th1 Cells. J Immunol. 2011;187:1157–1165. - PubMed
-
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. - PubMed
-
- Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

