HIV-1 gp120 vaccine induces affinity maturation in both new and persistent antibody clonal lineages
- PMID: 22553329
- PMCID: PMC3416280
- DOI: 10.1128/JVI.00426-12
HIV-1 gp120 vaccine induces affinity maturation in both new and persistent antibody clonal lineages
Abstract
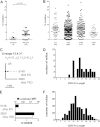
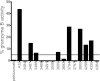
Most antibodies that broadly neutralize HIV-1 are highly somatically mutated in antibody clonal lineages that persist over time. Here, we describe the analysis of human antibodies induced during an HIV-1 vaccine trial (GSK PRO HIV-002) that used the clade B envelope (Env) gp120 of clone W6.1D (gp120(W6.1D)). Using dual-color antigen-specific sorting, we isolated Env-specific human monoclonal antibodies (MAbs) and studied the clonal persistence of antibodies in the setting of HIV-1 Env vaccination. We found evidence of V(H) somatic mutation induced by the vaccine but only to a modest level (3.8% ± 0.5%; range 0 to 8.2%). Analysis of 34 HIV-1-reactive MAbs recovered over four immunizations revealed evidence of both sequential recruitment of naïve B cells and restimulation of previously recruited memory B cells. These recombinant antibodies recapitulated the anti-HIV-1 activity of participant serum including pseudovirus neutralization and antibody-dependent cell-mediated cytotoxicity (ADCC). One antibody (3491) demonstrated a change in specificity following somatic mutation with binding of the inferred unmutated ancestor to a linear C2 peptide while the mutated antibody reacted only with a conformational epitope in gp120 Env. Thus, gp120(W6.1D) was strongly immunogenic but over four immunizations induced levels of affinity maturation below that of broadly neutralizing MAbs. Improved vaccination strategies will be needed to drive persistent stimulation of antibody clonal lineages to induce affinity maturation that results in highly mutated HIV-1 Env-reactive antibodies.
Figures




Similar articles
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
Structural Comparison of Human Anti-HIV-1 gp120 V3 Monoclonal Antibodies of the Same Gene Usage Induced by Vaccination and Chronic Infection.J Virol. 2018 Aug 29;92(18):e00641-18. doi: 10.1128/JVI.00641-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29997214 Free PMC article.
-
VH1-69 Utilizing Antibodies Are Capable of Mediating Non-neutralizing Fc-Mediated Effector Functions Against the Transmitted/Founder gp120.Front Immunol. 2019 Jan 15;9:3163. doi: 10.3389/fimmu.2018.03163. eCollection 2018. Front Immunol. 2019. PMID: 30697215 Free PMC article.
-
Structural Features of Broadly Neutralizing Antibodies and Rational Design of Vaccine.Adv Exp Med Biol. 2018;1075:73-95. doi: 10.1007/978-981-13-0484-2_4. Adv Exp Med Biol. 2018. PMID: 30030790 Review.
-
Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies.Immunity. 2012 Sep 21;37(3):412-25. doi: 10.1016/j.immuni.2012.08.012. Immunity. 2012. PMID: 22999947 Free PMC article. Review.
Cited by
-
Novel directions in HIV-1 vaccines revealed from clinical trials.Curr Opin HIV AIDS. 2013 Sep;8(5):421-31. doi: 10.1097/COH.0b013e3283632c26. Curr Opin HIV AIDS. 2013. PMID: 23743791 Free PMC article. Review.
-
Immunisation with foamy virus Bet fusion proteins as novel strategy for HIV-1 epitope delivery.Immunol Res. 2013 May;56(1):61-72. doi: 10.1007/s12026-013-8387-x. Immunol Res. 2013. PMID: 23440699
-
Adjuvant-Dependent Enhancement of HIV Env-Specific Antibody Responses in Infant Rhesus Macaques.J Virol. 2018 Sep 26;92(20):e01051-18. doi: 10.1128/JVI.01051-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30089691 Free PMC article.
-
Mixed Origins: HIV gp120-Specific Memory Develops from Pre-Existing Memory and Naive B Cells Following Vaccination in Humans.AIDS Res Hum Retroviruses. 2023 Jul;39(7):350-366. doi: 10.1089/AID.2022.0104. Epub 2023 Mar 22. AIDS Res Hum Retroviruses. 2023. PMID: 36762930 Free PMC article.
-
Elicitation of HIV-1-neutralizing antibodies against the CD4-binding site.Curr Opin HIV AIDS. 2013 Sep;8(5):382-92. doi: 10.1097/COH.0b013e328363a90e. Curr Opin HIV AIDS. 2013. PMID: 23924998 Free PMC article. Review.
References
-
- Abu-Shakra M, Press J, Buskila D, Sukenik S. 2007. Influenza vaccination of patients with systemic lupus erythematosus: safety and immunogenicity issues. Autoimmun. Rev. 6:543–546 - PubMed
-
- Batista FD, Neuberger MS. 1998. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity 8:751–759 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

