LuIII parvovirus selectively and efficiently targets, replicates in, and kills human glioma cells
- PMID: 22553327
- PMCID: PMC3416325
- DOI: 10.1128/JVI.00227-12
LuIII parvovirus selectively and efficiently targets, replicates in, and kills human glioma cells
Abstract
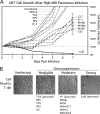
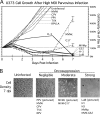
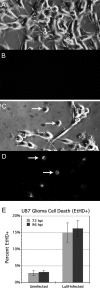
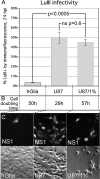
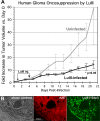
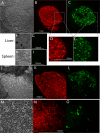
Because productive infection by parvoviruses requires cell division and is enhanced by oncogenic transformation, some parvoviruses may have potential utility in killing cancer cells. To identify the parvovirus(es) with the optimal oncolytic effect against human glioblastomas, we screened 12 parvoviruses at a high multiplicity of infection (MOI). MVMi, MVMc, MVM-G17, tumor virus X (TVX), canine parvovirus (CPV), porcine parvovirus (PPV), rat parvovirus 1A (RPV1A), and H-3 were relatively ineffective. The four viruses with the greatest oncolytic activity, LuIII, H-1, MVMp, and MVM-G52, were tested for the ability, at a low MOI, to progressively infect the culture over time, causing cell death at a rate higher than that of cell proliferation. LuIII alone was effective in all five human glioblastomas tested. H-1 progressively infected only two of five; MVMp and MVM-G52 were ineffective in all five. To investigate the underlying mechanism of LuIII's phenotype, we used recombinant parvoviruses with the LuIII capsid replacing the MVMp capsid or with molecular alteration of the P4 promoter. The LuIII capsid enhanced efficient replication and oncolysis in MO59J gliomas cells; other gliomas tested required the entire LuIII genome to exhibit enhanced infection. LuIII selectively infected glioma cells over normal glial cells in vitro. In mouse models, human glioblastoma xenografts were selectively infected by LuIII when administered intratumorally; LuIII reduced tumor growth by 75%. LuIII also had the capacity to selectively infect subcutaneous or intracranial gliomas after intravenous inoculation. Intravenous or intracranial LuIII caused no adverse effects. Intracranial LuIII caused no infection of mature mouse neurons or glia in vivo but showed a modest infection of developing neurons.
Figures



Similar articles
-
Oncolytic parvoviruses: from basic virology to clinical applications.Virol J. 2015 Jan 29;12:6. doi: 10.1186/s12985-014-0223-y. Virol J. 2015. PMID: 25630937 Free PMC article. Review.
-
Autonomous parvoviruses neither stimulate nor are inhibited by the type I interferon response in human normal or cancer cells.J Virol. 2014 May;88(9):4932-42. doi: 10.1128/JVI.03508-13. Epub 2014 Feb 19. J Virol. 2014. PMID: 24554651 Free PMC article.
-
Atomic Resolution Structure of the Oncolytic Parvovirus LuIII by Electron Microscopy and 3D Image Reconstruction.Viruses. 2017 Oct 30;9(11):321. doi: 10.3390/v9110321. Viruses. 2017. PMID: 29084163 Free PMC article.
-
Distinct host cell fates for human malignant melanoma targeted by oncolytic rodent parvoviruses.Virology. 2013 Nov;446(1-2):37-48. doi: 10.1016/j.virol.2013.07.013. Epub 2013 Aug 9. Virology. 2013. PMID: 24074565 Free PMC article.
-
Cancer gene therapy through autonomous parvovirus--mediated gene transfer.Curr Gene Ther. 2004 Sep;4(3):249-61. doi: 10.2174/1566523043346228. Curr Gene Ther. 2004. PMID: 15384939 Review.
Cited by
-
Antiangiogenic Vascular Endothelial Growth Factor-Blocking Peptides Displayed on the Capsid of an Infectious Oncolytic Parvovirus: Assembly and Immune Interactions.J Virol. 2019 Sep 12;93(19):e00798-19. doi: 10.1128/JVI.00798-19. Print 2019 Oct 1. J Virol. 2019. PMID: 31315994 Free PMC article.
-
Parvovirus Capsid-Antibody Complex Structures Reveal Conservation of Antigenic Epitopes Across the Family.Viral Immunol. 2021 Jan-Feb;34(1):3-17. doi: 10.1089/vim.2020.0022. Epub 2020 Apr 21. Viral Immunol. 2021. PMID: 32315582 Free PMC article. Review.
-
Intracellular virion traffic to the endosome driven by cell type specific sialic acid receptors determines parvovirus tropism.Front Microbiol. 2023 Jan 23;13:1063706. doi: 10.3389/fmicb.2022.1063706. eCollection 2022. Front Microbiol. 2023. PMID: 36756201 Free PMC article.
-
Tutorial: design, production and testing of oncolytic viruses for cancer immunotherapy.Nat Protoc. 2024 Sep;19(9):2540-2570. doi: 10.1038/s41596-024-00985-1. Epub 2024 May 20. Nat Protoc. 2024. PMID: 38769145 Review.
-
Oncolytic parvoviruses: from basic virology to clinical applications.Virol J. 2015 Jan 29;12:6. doi: 10.1186/s12985-014-0223-y. Virol J. 2015. PMID: 25630937 Free PMC article. Review.
References
-
- Abschuetz A, et al. 2006. Oncolytic murine autonomous parvovirus, a candidate vector for glioma gene therapy, is innocuous to normal and immunocompetent mouse glial cells. Cell Tissue Res. 325:423–436 - PubMed
-
- Arruda VR, Xiao W. 2007. It's all about the clothing: capsid domination in the adeno-associated viral vector world. J. Thromb. Haemost. 5:12–15 - PubMed
-
- Arstila P. 1976. Quantitative studies on adsorption, elution, and haemagglutination of vesicular stomatitis virus. Arch. Virol. 51:51–58 - PubMed
-
- Besselsen DG, Romero MJ, Wagner AM, Henderson KS, Livingston RS. 2006. Identification of novel murine parvovirus strains by epidemiological analysis of naturally infected mice. J. Gen. Virol. 87:1543–1556 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

