Role of deleted in breast cancer 1 (DBC1) protein in SIRT1 deacetylase activation induced by protein kinase A and AMP-activated protein kinase
- PMID: 22553202
- PMCID: PMC3390625
- DOI: 10.1074/jbc.M112.365874
Role of deleted in breast cancer 1 (DBC1) protein in SIRT1 deacetylase activation induced by protein kinase A and AMP-activated protein kinase
Abstract
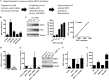
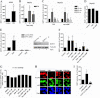
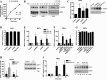
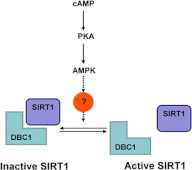
The NAD(+)-dependent deacetylase SIRT1 is a key regulator of several aspects of metabolism and aging. SIRT1 activation is beneficial for several human diseases, including metabolic syndrome, diabetes, obesity, liver steatosis, and Alzheimer disease. We have recently shown that the protein deleted in breast cancer 1 (DBC1) is a key regulator of SIRT1 activity in vivo. Furthermore, SIRT1 and DBC1 form a dynamic complex that is regulated by the energetic state of the organism. Understanding how the interaction between SIRT1 and DBC1 is regulated is therefore essential to design strategies aimed to activate SIRT1. Here, we investigated which pathways can lead to the dissociation of SIRT1 and DBC1 and consequently to SIRT1 activation. We observed that PKA activation leads to a fast and transient activation of SIRT1 that is DBC1-dependent. In fact, an increase in cAMP/PKA activity resulted in the dissociation of SIRT1 and DBC1 in an AMP-activated protein kinase (AMPK)-dependent manner. Pharmacological AMPK activation led to SIRT1 activation by a DBC1-dependent mechanism. Indeed, we found that AMPK activators promote SIRT1-DBC1 dissociation in cells, resulting in an increase in SIRT1 activity. In addition, we observed that the SIRT1 activation promoted by PKA and AMPK occurs without changes in the intracellular levels of NAD(+). We propose that PKA and AMPK can acutely activate SIRT1 by inducing dissociation of SIRT1 from its endogenous inhibitor DBC1. Our experiments provide new insight on the in vivo mechanism of SIRT1 regulation and a new avenue for the development of pharmacological SIRT1 activators targeted at the dissociation of the SIRT1-DBC1 complex.
Figures




Similar articles
-
Inhibition of NAMPT aggravates high fat diet-induced hepatic steatosis in mice through regulating Sirt1/AMPKα/SREBP1 signaling pathway.Lipids Health Dis. 2017 Apr 27;16(1):82. doi: 10.1186/s12944-017-0464-z. Lipids Health Dis. 2017. PMID: 28449683 Free PMC article.
-
Resveratrol-Induced AMP-Activated Protein Kinase Activation Is Cell-Type Dependent: Lessons from Basic Research for Clinical Application.Nutrients. 2017 Jul 14;9(7):751. doi: 10.3390/nu9070751. Nutrients. 2017. PMID: 28708087 Free PMC article.
-
Deleted in breast cancer-1 regulates SIRT1 activity and contributes to high-fat diet-induced liver steatosis in mice.J Clin Invest. 2010 Feb;120(2):545-58. doi: 10.1172/JCI39319. Epub 2010 Jan 11. J Clin Invest. 2010. PMID: 20071779 Free PMC article.
-
Biochemical effects of SIRT1 activators.Biochim Biophys Acta. 2010 Aug;1804(8):1626-34. doi: 10.1016/j.bbapap.2009.10.025. Epub 2009 Nov 6. Biochim Biophys Acta. 2010. PMID: 19897059 Free PMC article. Review.
-
Nutraceutical activation of Sirt1: a review.Open Heart. 2022 Dec;9(2):e002171. doi: 10.1136/openhrt-2022-002171. Open Heart. 2022. PMID: 36522127 Free PMC article. Review.
Cited by
-
Skeletal muscle SIRT1 and the genetics of metabolic health: therapeutic activation by pharmaceuticals and exercise.Appl Clin Genet. 2012 Aug 29;5:81-91. doi: 10.2147/TACG.S31276. Print 2012. Appl Clin Genet. 2012. PMID: 23776383 Free PMC article.
-
Mechanistic insights into the dual role of CCAR2/DBC1 in cancer.Exp Mol Med. 2023 Aug;55(8):1691-1701. doi: 10.1038/s12276-023-01058-1. Epub 2023 Aug 1. Exp Mol Med. 2023. PMID: 37524873 Free PMC article. Review.
-
Specific Sirt1 Activator-mediated Improvement in Glucose Homeostasis Requires Sirt1-Independent Activation of AMPK.EBioMedicine. 2017 Apr;18:128-138. doi: 10.1016/j.ebiom.2017.03.019. Epub 2017 Mar 14. EBioMedicine. 2017. PMID: 28396013 Free PMC article.
-
Resveratrol as a calorie restriction mimetic: therapeutic implications.Trends Cell Biol. 2012 Oct;22(10):546-54. doi: 10.1016/j.tcb.2012.07.004. Epub 2012 Aug 10. Trends Cell Biol. 2012. PMID: 22885100 Free PMC article. Review.
-
Regulation of anoikis by deleted in breast cancer-1 (DBC1) through NF-κB.Apoptosis. 2013 Aug;18(8):949-62. doi: 10.1007/s10495-013-0847-1. Apoptosis. 2013. PMID: 23588592 Free PMC article.
References
-
- Rodgers J. T., Lerin C., Haas W., Gygi S. P., Spiegelman B. M., Puigserver P. (2005) Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 - PubMed
-
- Moynihan K. A., Grimm A. A., Plueger M. M., Bernal-Mizrachi E., Ford E., Cras-Mneur C., Permutt M. A., Imai S. (2005) Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2, 105–117 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

