Astrocytes and disease: a neurodevelopmental perspective
- PMID: 22549954
- PMCID: PMC3347787
- DOI: 10.1101/gad.188326.112
Astrocytes and disease: a neurodevelopmental perspective
Erratum in
- Genes Dev. 2012 Jul 1;26(13):1508. Krenick, Robert [corrected to Krencik, Robert]; Ullian, Erik [corrected to Ullian, Erik M]
Abstract
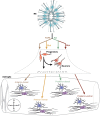
Astrocytes are no longer seen as a homogenous population of cells. In fact, recent studies indicate that astrocytes are morphologically and functionally diverse and play critical roles in neurodevelopmental diseases such as Rett syndrome and fragile X mental retardation. This review summarizes recent advances in astrocyte development, including the role of neural tube patterning in specification and developmental functions of astrocytes during synaptogenesis. We propose here that a precise understanding of astrocyte development is critical to defining heterogeneity and could lead advances in understanding and treating a variety of neuropsychiatric diseases.
Figures
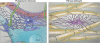


Similar articles
-
Astrocyte development: A Guide for the Perplexed.Glia. 2015 Aug;63(8):1320-9. doi: 10.1002/glia.22836. Epub 2015 May 12. Glia. 2015. PMID: 25963996 Review.
-
Astrocyte Diversity: Current Insights and Future Directions.Neurochem Res. 2020 Jun;45(6):1298-1305. doi: 10.1007/s11064-020-02959-7. Epub 2020 Jan 31. Neurochem Res. 2020. PMID: 32006215 Free PMC article. Review.
-
Do Astrocytes Play a Role in Intellectual Disabilities?Trends Neurosci. 2019 Aug;42(8):518-527. doi: 10.1016/j.tins.2019.05.011. Epub 2019 Jul 9. Trends Neurosci. 2019. PMID: 31300246 Review.
-
Origin, molecular specification, and stemness of astrocytes.Dev Neurobiol. 2022 Mar;82(2):149-159. doi: 10.1002/dneu.22863. Epub 2022 Feb 2. Dev Neurobiol. 2022. PMID: 35006642 Review.
-
Neurorestorative Role of Stem Cells in Alzheimer's Disease: Astrocyte Involvement.Curr Alzheimer Res. 2016;13(4):419-27. doi: 10.2174/156720501304160314162812. Curr Alzheimer Res. 2016. PMID: 27018261 Review.
Cited by
-
Human pluripotent stem cell-derived radial glia recapitulate developmental events and provide real-time access to cortical neurons and astrocytes.Stem Cells Transl Med. 2015 May;4(5):437-47. doi: 10.5966/sctm.2014-0137. Epub 2015 Apr 1. Stem Cells Transl Med. 2015. PMID: 25834120 Free PMC article.
-
Building blocks of the cerebral cortex: from development to the dish.Wiley Interdiscip Rev Dev Biol. 2015 Sep-Oct;4(5):529-44. doi: 10.1002/wdev.192. Epub 2015 Apr 29. Wiley Interdiscip Rev Dev Biol. 2015. PMID: 25926310 Free PMC article. Review.
-
Developmental neurotoxicity of 3,3',4,4'-tetrachloroazobenzene with thyroxine deficit: Sensitivity of glia and dentate granule neurons in the absence of behavioral changes.Toxics. 2014 Sep;2(3):496-532. doi: 10.3390/toxics2030496. Toxics. 2014. PMID: 26029700 Free PMC article.
-
Regenerating white matter using human iPSC-derived immature astroglia.Neurogenesis (Austin). 2016 Sep 8;3(1):e1224453. doi: 10.1080/23262133.2016.1224453. eCollection 2016. Neurogenesis (Austin). 2016. PMID: 27652287 Free PMC article.
-
Astrocytic LRRK2 Controls Synaptic Connectivity via Regulation of ERM Phosphorylation.bioRxiv [Preprint]. 2024 Aug 28:2023.04.09.536178. doi: 10.1101/2023.04.09.536178. bioRxiv. 2024. PMID: 39253496 Free PMC article. Preprint.
References
-
- Agulhon C, Fiacco TA, McCarthy KD 2010. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327: 1250–1254 - PubMed
-
- Allaman I, Bélanger M, Magistretti PJ 2011. Astrocyte–neuron metabolic relationships: For better and for worse. Trends Neurosci 34: 76–87 - PubMed
-
- Allen NJ, Chakraborty C, Howe ML, Barres BA 2011. Identification of an astrocyte-derived factor that promotes the formation of excitatory synapses containing GluA1 AMPA glutamate receptors. Program no. 436.10/A61. Neuroscience Meeting Planner. Society for Neuroscience, Washington, DC. Online.
-
- Altshuler LL, Abulseoud OA, Foland-Ross L, Bartzokis G, Chang S, Mintz J, Hellemann G, Vinters HV 2010. Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disord 12: 541–549 - PubMed
-
- Andersson E, Jensen JB, Parmar M, Guillemot F, Björklund A 2006. Development of the mesencephalic dopaminergic neuron system is compromised in the absence of neurogenin 2. Development 133: 507–516 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
