Sialyllactose in viral membrane gangliosides is a novel molecular recognition pattern for mature dendritic cell capture of HIV-1
- PMID: 22545022
- PMCID: PMC3335875
- DOI: 10.1371/journal.pbio.1001315
Sialyllactose in viral membrane gangliosides is a novel molecular recognition pattern for mature dendritic cell capture of HIV-1
Abstract
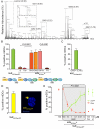
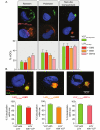
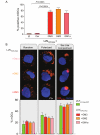
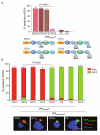
HIV-1 is internalized into mature dendritic cells (mDCs) via an as yet undefined mechanism with subsequent transfer of stored, infectious virus to CD4+ T lymphocytes. Thus, HIV-1 subverts a DC antigen capture mechanism to promote viral spread. Here, we show that gangliosides in the HIV-1 membrane are the key molecules for mDC uptake. HIV-1 virus-like particles and liposomes mimicking the HIV-1 lipid composition were shown to use a common internalization pathway and the same trafficking route within mDCs. Hence, these results demonstrate that gangliosides can act as viral attachment factors, in addition to their well known function as cellular receptors for certain viruses. Furthermore, the sialyllactose molecule present in specific gangliosides was identified as the determinant moiety for mDC HIV-1 uptake. Thus, sialyllactose represents a novel molecular recognition pattern for mDC capture, and may be crucial both for antigen presentation leading to immunity against pathogens and for succumbing to subversion by HIV-1.
Conflict of interest statement
A patent application based on this work has been filed (EP11382392.6, 2011). The authors have declared that no other competing interests exist.
Figures


Comment in
-
Lipids in HIV's envelope help the virus to spread.PLoS Biol. 2012 Apr;10(4):e1001316. doi: 10.1371/journal.pbio.1001316. Epub 2012 Apr 24. PLoS Biol. 2012. PMID: 22563298 Free PMC article.
Similar articles
-
Lipids in HIV's envelope help the virus to spread.PLoS Biol. 2012 Apr;10(4):e1001316. doi: 10.1371/journal.pbio.1001316. Epub 2012 Apr 24. PLoS Biol. 2012. PMID: 22563298 Free PMC article.
-
Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides.PLoS Biol. 2012;10(12):e1001448. doi: 10.1371/journal.pbio.1001448. Epub 2012 Dec 18. PLoS Biol. 2012. PMID: 23271952 Free PMC article.
-
Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway.Blood. 2009 Mar 19;113(12):2732-41. doi: 10.1182/blood-2008-05-158642. Epub 2008 Oct 22. Blood. 2009. PMID: 18945959 Free PMC article.
-
HIV-1 capture and transmission by dendritic cells: the role of viral glycolipids and the cellular receptor Siglec-1.PLoS Pathog. 2014 Jul 17;10(7):e1004146. doi: 10.1371/journal.ppat.1004146. eCollection 2014 Jul. PLoS Pathog. 2014. PMID: 25033082 Free PMC article. Review.
-
Viral piracy: HIV-1 targets dendritic cells for transmission.Curr HIV Res. 2006 Apr;4(2):169-76. doi: 10.2174/157016206776055020. Curr HIV Res. 2006. PMID: 16611055 Review.
Cited by
-
Dressing up Nanoparticles: A Membrane Wrap to Induce Formation of the Virological Synapse.ACS Nano. 2015;9(4):4182-92. doi: 10.1021/acsnano.5b00415. Epub 2015 Apr 14. ACS Nano. 2015. PMID: 25853367 Free PMC article.
-
Virus-Mimicking Polymer Nanoparticles Targeting CD169+ Macrophages as Long-Acting Nanocarriers for Combination Antiretrovirals.ACS Appl Mater Interfaces. 2022 Jan 19;14(2):2488-2500. doi: 10.1021/acsami.1c17415. Epub 2022 Jan 7. ACS Appl Mater Interfaces. 2022. PMID: 34995059 Free PMC article.
-
Glycosphingolipid-functionalized nanoparticles recapitulate CD169-dependent HIV-1 uptake and trafficking in dendritic cells.Nat Commun. 2014 Jun 20;5:4136. doi: 10.1038/ncomms5136. Nat Commun. 2014. PMID: 24947940 Free PMC article.
-
Quantifying lipid contents in enveloped virus particles with plasmonic nanoparticles.Small. 2015 Apr;11(13):1592-602. doi: 10.1002/smll.201402184. Epub 2014 Nov 10. Small. 2015. PMID: 25382201 Free PMC article.
-
Uncloaking the viral glycocalyx: How do viruses exploit glycoimmune checkpoints?Adv Virus Res. 2024;119:63-110. doi: 10.1016/bs.aivir.2024.03.001. Epub 2024 Apr 8. Adv Virus Res. 2024. PMID: 38897709 Free PMC article. Review.
References
-
- Banchereau J, Steinman R. M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Steinman R. M, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. - PubMed
-
- Mellman I, Steinman R. M. Dendritic cells specialized and regulated antigen processing machines. Cell. 2001;106:255–258. - PubMed
-
- Villadangos J. A, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. - PubMed
-
- Probst H. C, van den Broek M. Priming of CTLs by lymphocytic choriomeningitis virus depends on dendritic cells. J Immunol. 2005;174:3920–3924. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

