Hepatitis C virus attachment mediated by apolipoprotein E binding to cell surface heparan sulfate
- PMID: 22532692
- PMCID: PMC3416335
- DOI: 10.1128/JVI.07222-11
Hepatitis C virus attachment mediated by apolipoprotein E binding to cell surface heparan sulfate
Abstract
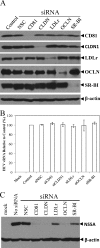
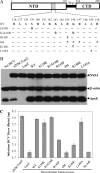
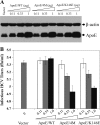
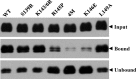
Viruses are known to use virally encoded envelope proteins for cell attachment, which is the very first step of virus infection. In the present study, we have obtained substantial evidence demonstrating that hepatitis C virus (HCV) uses the cellular protein apolipoprotein E (apoE) for its attachment to cells. An apoE-specific monoclonal antibody was able to efficiently block HCV attachment to the hepatoma cell line Huh-7.5 as well as primary human hepatocytes. After HCV bound to cells, however, anti-apoE antibody was unable to inhibit virus infection. Conversely, the HCV E2-specific monoclonal antibody CBH5 did not affect HCV attachment but potently inhibited HCV entry. Similarly, small interfering RNA-mediated knockdown of the key HCV receptor/coreceptor molecules CD81, claudin-1, low-density lipoprotein receptor (LDLr), occludin, and SR-BI did not affect HCV attachment but efficiently suppressed HCV infection, suggesting their important roles in HCV infection at postattachment steps. Strikingly, removal of heparan sulfate from the cell surface by treatment with heparinase blocked HCV attachment. Likewise, substitutions of the positively charged amino acids with neutral or negatively charged residues in the receptor-binding region of apoE resulted in a reduction of apoE-mediating HCV infection. More importantly, mutations of the arginine and lysine to alanine or glutamic acid in the receptor-binding region ablated the heparin-binding activity of apoE, as determined by an in vitro heparin pulldown assay. HCV attachment could also be inhibited by a synthetic peptide derived from the apoE receptor-binding region. Collectively, these findings demonstrate that apoE mediates HCV attachment through specific interactions with cell surface heparan sulfate.
Figures

Similar articles
-
Apolipoprotein E mediates attachment of clinical hepatitis C virus to hepatocytes by binding to cell surface heparan sulfate proteoglycan receptors.PLoS One. 2013 Jul 2;8(7):e67982. doi: 10.1371/journal.pone.0067982. Print 2013. PLoS One. 2013. PMID: 23844141 Free PMC article.
-
Attachment and Postattachment Receptors Important for Hepatitis C Virus Infection and Cell-to-Cell Transmission.J Virol. 2017 Jun 9;91(13):e00280-17. doi: 10.1128/JVI.00280-17. Print 2017 Jul 1. J Virol. 2017. PMID: 28404852 Free PMC article.
-
Characterization of hepatitis C virus interaction with heparan sulfate proteoglycans.J Virol. 2015 Apr;89(7):3846-58. doi: 10.1128/JVI.03647-14. Epub 2015 Jan 21. J Virol. 2015. PMID: 25609801 Free PMC article.
-
The Role of ApoE in HCV Infection and Comorbidity.Int J Mol Sci. 2019 Apr 25;20(8):2037. doi: 10.3390/ijms20082037. Int J Mol Sci. 2019. PMID: 31027190 Free PMC article. Review.
-
Hepatitis C virus cell entry: role of lipoproteins and cellular receptors.J Gen Virol. 2009 May;90(Pt 5):1055-1070. doi: 10.1099/vir.0.008300-0. Epub 2009 Mar 4. J Gen Virol. 2009. PMID: 19264629 Review.
Cited by
-
Genetic Diversity Underlying the Envelope Glycoproteins of Hepatitis C Virus: Structural and Functional Consequences and the Implications for Vaccine Design.Viruses. 2015 Jul 17;7(7):3995-4046. doi: 10.3390/v7072809. Viruses. 2015. PMID: 26193307 Free PMC article. Review.
-
Lipids and HCV.Semin Immunopathol. 2013 Jan;35(1):87-100. doi: 10.1007/s00281-012-0356-2. Epub 2012 Oct 31. Semin Immunopathol. 2013. PMID: 23111699 Review.
-
Functional and Biochemical Characterization of Hepatitis C Virus (HCV) Particles Produced in a Humanized Liver Mouse Model.J Biol Chem. 2015 Sep 18;290(38):23173-87. doi: 10.1074/jbc.M115.662999. Epub 2015 Jul 29. J Biol Chem. 2015. PMID: 26224633 Free PMC article.
-
Extracellular lipid-free apolipoprotein E inhibits HCV replication and induces ABCG1-dependent cholesterol efflux.Gut. 2017 May;66(5):896-907. doi: 10.1136/gutjnl-2015-311289. Epub 2016 Sep 8. Gut. 2017. PMID: 27609828 Free PMC article.
-
Apolipoprotein E is an HIV-1-inducible inhibitor of viral production and infectivity in macrophages.PLoS Pathog. 2018 Nov 29;14(11):e1007372. doi: 10.1371/journal.ppat.1007372. eCollection 2018 Nov. PLoS Pathog. 2018. PMID: 30496280 Free PMC article.
References
-
- Acharya P, et al. 2002. Comparison of the stabilities and unfolding pathways of human apolipoprotein E isoforms by differential scanning calorimetry and circular dichroism. Biochim. Biophys. Acta 1584:9–19 - PubMed
-
- Alter MJ. 2006. Epidemiology of viral hepatitis and HIV co-infection. J. Hepatol. 44:S6–S9 - PubMed
-
- André P, Perlemuter G, Budkowska A, Brechot C, Lotteau V. 2005. Hepatitis C virus particles and lipoprotein metabolism. Semin. Liver Dis. 25:93–104 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

