Microbicidal activity of vascular peroxidase 1 in human plasma via generation of hypochlorous acid
- PMID: 22526679
- PMCID: PMC3416459
- DOI: 10.1128/IAI.06337-11
Microbicidal activity of vascular peroxidase 1 in human plasma via generation of hypochlorous acid
Abstract
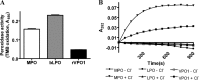
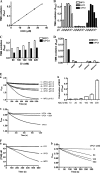
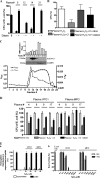
Members of the heme peroxidase family play an important role in host defense. Myeloperoxidase (MPO) is expressed in phagocytes and is the only animal heme peroxidase previously reported to be capable of using chloride ion as a substrate to form the highly microbicidal species hypochlorous acid (HOCl) at neutral pH. Despite the potent bacterial killing activity of HOCl, individuals who fail to express MPO typically show only a modest increase in some fungal infections. This may point to the existence of redundant host defense mechanisms. Vascular peroxidase 1 (VPO1) is newly discovered member of the heme peroxidase family. VPO1 is expressed in cells of the cardiovascular system and is secreted into the bloodstream. In the present study, we investigate whether VPO1 is capable of generating HOCl and its role in host defense. Like MPO, VPO1 in the presence of H₂O₂ and chloride generates HOCl. VPO1-dependent HOCl generation was demonstrated by chlorination of taurine and tyrosine using mass spectrometry. In addition, the VPO1/H₂O₂/Cl⁻ system can cause the chlorination of monochlorodimedone and the oxidation of 5-thio-2-nitrobenzoic acid. Purified VPO1 and VPO1 in plasma mediate bacterial killing that is dependent on chloride and H₂O₂; killing is inhibited by peroxidase inhibitors and by the H₂O₂ scavenger catalase. In the presence of erythrocytes, bacterial killing by VPO1 is slightly reduced. Thus, VPO1, in addition to MPO, is the second member of the heme peroxidase family capable of generating HOCl under physiological conditions. VPO1 is likely to participate in host defense, with bactericidal activity mediated through the generation of HOCl.
Figures

Similar articles
-
Vascular peroxidase 1 catalyzes the formation of hypohalous acids: characterization of its substrate specificity and enzymatic properties.Free Radic Biol Med. 2012 Nov 15;53(10):1954-9. doi: 10.1016/j.freeradbiomed.2012.08.597. Epub 2012 Sep 12. Free Radic Biol Med. 2012. PMID: 22982576 Free PMC article.
-
Involvement of vascular peroxidase 1 in angiotensin II-induced hypertrophy of H9c2 cells.J Am Soc Hypertens. 2017 Aug;11(8):519-529.e1. doi: 10.1016/j.jash.2016.08.002. Epub 2016 Aug 24. J Am Soc Hypertens. 2017. PMID: 27651140
-
The role of vascular peroxidase 1 in ox-LDL-induced vascular smooth muscle cell calcification.Atherosclerosis. 2015 Dec;243(2):357-63. doi: 10.1016/j.atherosclerosis.2015.08.047. Epub 2015 Sep 3. Atherosclerosis. 2015. PMID: 26520887
-
Vascular peroxidase 1: a novel enzyme in promoting oxidative stress in cardiovascular system.Trends Cardiovasc Med. 2013 Jul;23(5):179-83. doi: 10.1016/j.tcm.2012.11.002. Epub 2013 Jan 26. Trends Cardiovasc Med. 2013. PMID: 23357484 Review.
-
Bacterial Defense Systems against the Neutrophilic Oxidant Hypochlorous Acid.Infect Immun. 2020 Jun 22;88(7):e00964-19. doi: 10.1128/IAI.00964-19. Print 2020 Jun 22. Infect Immun. 2020. PMID: 32152198 Free PMC article. Review.
Cited by
-
Recent Progress in Metal-Organic Framework (MOF) Based Luminescent Chemodosimeters.Nanomaterials (Basel). 2019 Jul 3;9(7):974. doi: 10.3390/nano9070974. Nanomaterials (Basel). 2019. PMID: 31277318 Free PMC article. Review.
-
Vascular peroxidase 1 catalyzes the formation of hypohalous acids: characterization of its substrate specificity and enzymatic properties.Free Radic Biol Med. 2012 Nov 15;53(10):1954-9. doi: 10.1016/j.freeradbiomed.2012.08.597. Epub 2012 Sep 12. Free Radic Biol Med. 2012. PMID: 22982576 Free PMC article.
-
Myeloperoxidase-Related Chlorination Activity Is Positively Associated with Circulating Ceruloplasmin in Chronic Heart Failure Patients: Relationship with Neurohormonal, Inflammatory, and Nutritional Parameters.Biomed Res Int. 2015;2015:691693. doi: 10.1155/2015/691693. Epub 2015 Oct 11. Biomed Res Int. 2015. PMID: 26539521 Free PMC article.
-
Folic acid modulates VPO1 DNA methylation levels and alleviates oxidative stress-induced apoptosis in vivo and in vitro.Redox Biol. 2018 Oct;19:81-91. doi: 10.1016/j.redox.2018.08.005. Epub 2018 Aug 8. Redox Biol. 2018. PMID: 30125807 Free PMC article.
-
Myeloperoxidase-dependent LDL modifications in bloodstream are mainly predicted by angiotensin II, adiponectin, and myeloperoxidase activity: a cross-sectional study in men.Mediators Inflamm. 2013;2013:750742. doi: 10.1155/2013/750742. Epub 2013 Nov 18. Mediators Inflamm. 2013. PMID: 24347830 Free PMC article.
References
-
- Arnhold J, Flemmig J. 2010. Human myeloperoxidase in innate and acquired immunity. Arch. Biochem. Biophys. 500:92–106 - PubMed
-
- Chan JR, Hyduk SJ, Cybulsky MI. 2000. Alpha 4 beta 1 integrin/VCAM-1 interaction activates α-l-β2-integrin-mediated adhesion to ICAM-1 in human T cells. J. Immunol. 164:746–753 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

