Analysis of a Jup hypomorphic allele reveals a critical threshold for postnatal viability
- PMID: 22522917
- PMCID: PMC3410040
- DOI: 10.1002/dvg.22034
Analysis of a Jup hypomorphic allele reveals a critical threshold for postnatal viability
Abstract
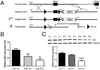
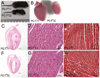
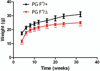
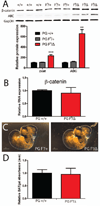
Mutations in the human Jup gene cause arrhythmogenic right ventricular cardiomyopathy (ARVC), a heart muscle disease that often leads to sudden cardiac death. Inactivation of the murine Jup gene (also known as plakoglobin) results in embryonic lethality due to cardiac rupture. In an effort to generate a conditional knockout allele, a neomycin cassette was introduced into the murine plakoglobin (PG) gene. This allele (PG F(N)) functions as a hypomorph when combined with a null allele (PG Δ). About half of the PG F(N)/Δ animals were smaller than their littermates and died before weaning age, whereas the remaining PG F(N)/Δ animals survived. Despite the reduced levels of PG in the heart, there were no signs of cardiomyopathy or cardiac dysfunction as determined by echocardiography. Importantly, the PG homolog, β-catenin (CTNNB1), was increased in the PG F(N)/Δ hearts. In addition to its structural role as part of the N-cadherin/catenin adhesion complex, β-catenin is a downstream effector of Wnt signaling. However, no change in β-catenin/TCF reporter activity was observed in PG F(N)/Δ embryos suggesting that excess β-catenin was not likely causing increased transcription of Wnt/β-catenin target genes. These data suggest novel function(s) for PG beyond the heart and define a critical threshold of PG expression that is necessary for postnatal survival.
Copyright © 2012 Wiley Periodicals, Inc.
Figures



Similar articles
-
Beyond cell adhesion: the role of armadillo proteins in the heart.Cell Signal. 2013 Jan;25(1):93-100. doi: 10.1016/j.cellsig.2012.09.025. Epub 2012 Sep 27. Cell Signal. 2013. PMID: 23022961 Free PMC article. Review.
-
Loss of cadherin-binding proteins β-catenin and plakoglobin in the heart leads to gap junction remodeling and arrhythmogenesis.Mol Cell Biol. 2012 Mar;32(6):1056-67. doi: 10.1128/MCB.06188-11. Epub 2012 Jan 17. Mol Cell Biol. 2012. PMID: 22252313 Free PMC article.
-
Cardiac tissue-restricted deletion of plakoglobin results in progressive cardiomyopathy and activation of {beta}-catenin signaling.Mol Cell Biol. 2011 Mar;31(6):1134-44. doi: 10.1128/MCB.01025-10. Epub 2011 Jan 18. Mol Cell Biol. 2011. PMID: 21245375 Free PMC article.
-
Restrictive loss of plakoglobin in cardiomyocytes leads to arrhythmogenic cardiomyopathy.Hum Mol Genet. 2011 Dec 1;20(23):4582-96. doi: 10.1093/hmg/ddr392. Epub 2011 Aug 31. Hum Mol Genet. 2011. PMID: 21880664 Free PMC article.
-
The canonical way to make a heart: β-catenin and plakoglobin in heart development and remodeling.Exp Biol Med (Maywood). 2017 Dec;242(18):1735-1745. doi: 10.1177/1535370217732737. Epub 2017 Sep 18. Exp Biol Med (Maywood). 2017. PMID: 28920469 Free PMC article. Review.
Cited by
-
Normalization of Naxos plakoglobin levels restores cardiac function in mice.J Clin Invest. 2015 Apr;125(4):1708-12. doi: 10.1172/JCI80335. Epub 2015 Feb 23. J Clin Invest. 2015. PMID: 25705887 Free PMC article.
-
Understanding the molecular basis of cardiomyopathy.Am J Physiol Heart Circ Physiol. 2022 Feb 1;322(2):H181-H233. doi: 10.1152/ajpheart.00562.2021. Epub 2021 Nov 19. Am J Physiol Heart Circ Physiol. 2022. PMID: 34797172 Free PMC article. Review.
-
Towards a Better Understanding of Genotype-Phenotype Correlations and Therapeutic Targets for Cardiocutaneous Genes: The Importance of Functional Studies above Prediction.Int J Mol Sci. 2022 Sep 15;23(18):10765. doi: 10.3390/ijms231810765. Int J Mol Sci. 2022. PMID: 36142674 Free PMC article. Review.
-
Arrhythmogenic Cardiomyopathy: from Preclinical Models to Genotype-phenotype Correlation and Pathophysiology.Stem Cell Rev Rep. 2023 Nov;19(8):2683-2708. doi: 10.1007/s12015-023-10615-0. Epub 2023 Sep 20. Stem Cell Rev Rep. 2023. PMID: 37731079 Free PMC article. Review.
-
Beyond cell adhesion: the role of armadillo proteins in the heart.Cell Signal. 2013 Jan;25(1):93-100. doi: 10.1016/j.cellsig.2012.09.025. Epub 2012 Sep 27. Cell Signal. 2013. PMID: 23022961 Free PMC article. Review.
References
-
- Asimaki A, Tandri H, Huang H, Halushka MK, Gautam S, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, McKenna WJ, Calkins H, Saffitz JE. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360:1075–1084. - PubMed
-
- Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–1300. - PubMed
-
- Bauce B, Nava A, Beffagna G, Basso C, Lorenzon A, Smaniotto G, De Bortoli M, Rigato I, Mazzotti E, Steriotis A, Marra MP, Towbin JA, Thiene G, Danieli GA, Rampazzo A. Multiple mutations in desmosomal proteins encoding genes in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2010;7:22–29. - PubMed
-
- Bierkamp C, McLaughlin KJ, Schwarz H, Huber O, Kemler R. Embryonic heart and skin defects in mice lacking plakoglobin. Dev Biol. 1996;180:780–785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

