The trouble with spines in fragile X syndrome: density, maturity and plasticity
- PMID: 22522472
- PMCID: PMC3422423
- DOI: 10.1016/j.neuroscience.2012.03.049
The trouble with spines in fragile X syndrome: density, maturity and plasticity
Abstract
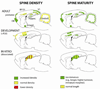
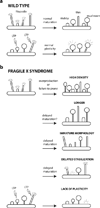
Dendritic spines are the principal recipients of excitatory synaptic inputs and the basic units of neural computation in the mammalian brain. Alterations in the density, size, shape, and turnover of mature spines, or defects in how spines are generated and establish synapses during brain development, could all result in neuronal dysfunction and lead to cognitive and/or behavioral impairments. That spines are abnormal in fragile X syndrome (FXS) and in the best-studied animal model of this disorder, the Fmr1 knockout mouse, is an undeniable fact. But the trouble with spines in FXS is that the exact nature of their defect is still controversial. Here, we argue that the most consistent abnormality of spines in FXS may be a subtle defect in activity-dependent spine plasticity and maturation. We also propose some future directions for research into spine plasticity in FXS at the cellular and ultrastructural levels that could help solve a two-decade-long riddle about the integrity of synapses in this prototypical neurodevelopmental disorder.
Keywords: Div; EM; FMRP; FXS; Fmr1; GFP; KO; L; LTD; LTP; P; WT; days in vitro; electron microscopy; filopodia; fragile X mental retardation protein; fragile X syndrome; green fluorescent protein; knockout; layer; long-term depression; long-term potentiation; mGluR; metabotropic glutamate receptor; postnatal day; synaptic plasticity; two-photon; wild-type.
Copyright © 2012 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Alterations in CA1 hippocampal synapses in a mouse model of fragile X syndrome.Glia. 2018 Apr;66(4):789-800. doi: 10.1002/glia.23284. Epub 2017 Dec 23. Glia. 2018. PMID: 29274095 Free PMC article.
-
Delayed stabilization of dendritic spines in fragile X mice.J Neurosci. 2010 Jun 9;30(23):7793-803. doi: 10.1523/JNEUROSCI.0577-10.2010. J Neurosci. 2010. PMID: 20534828 Free PMC article.
-
Fragile X-like behaviors and abnormal cortical dendritic spines in cytoplasmic FMR1-interacting protein 2-mutant mice.Hum Mol Genet. 2015 Apr 1;24(7):1813-23. doi: 10.1093/hmg/ddu595. Epub 2014 Nov 28. Hum Mol Genet. 2015. PMID: 25432536 Free PMC article.
-
BDNF in fragile X syndrome.Neuropharmacology. 2014 Jan;76 Pt C:729-36. doi: 10.1016/j.neuropharm.2013.05.018. Epub 2013 May 29. Neuropharmacology. 2014. PMID: 23727436 Review.
-
Which comes first in fragile X syndrome, dendritic spine dysgenesis or defects in circuit plasticity?Neuroscientist. 2012 Feb;18(1):28-44. doi: 10.1177/1073858410395322. Epub 2011 May 6. Neuroscientist. 2012. PMID: 21551076 Review.
Cited by
-
Seizures in Fragile X Syndrome: Associations and Longitudinal Analysis of a Large Clinic-Based Cohort.Front Pediatr. 2021 Dec 30;9:736255. doi: 10.3389/fped.2021.736255. eCollection 2021. Front Pediatr. 2021. PMID: 35036394 Free PMC article.
-
Altered Behavior in Mice Socially Isolated During Adolescence Corresponds With Immature Dendritic Spine Morphology and Impaired Plasticity in the Prefrontal Cortex.Front Behav Neurosci. 2018 May 9;12:87. doi: 10.3389/fnbeh.2018.00087. eCollection 2018. Front Behav Neurosci. 2018. PMID: 29867388 Free PMC article.
-
Cell-type-specific disruption of cortico-striatal circuitry drives repetitive patterns of behavior in fragile X syndrome model mice.Cell Rep. 2023 Aug 29;42(8):112901. doi: 10.1016/j.celrep.2023.112901. Epub 2023 Jul 27. Cell Rep. 2023. PMID: 37505982 Free PMC article.
-
Atypical retinal function in a mouse model of Fragile X syndrome.bioRxiv [Preprint]. 2024 Mar 17:2024.03.15.585283. doi: 10.1101/2024.03.15.585283. bioRxiv. 2024. PMID: 38559003 Free PMC article. Preprint.
-
Inhibition of phosphodiesterase-4D in adults with fragile X syndrome: a randomized, placebo-controlled, phase 2 clinical trial.Nat Med. 2021 May;27(5):862-870. doi: 10.1038/s41591-021-01321-w. Epub 2021 Apr 29. Nat Med. 2021. PMID: 33927413 Clinical Trial.
References
-
- Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci. 2006;32:37–48. - PubMed
-
- Bagni C, Greenough WT. From mRNP traficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nature Rev Neurosci. 2005;376:376–387. - PubMed
-
- Beltran-Campos V, Prado-Alcala RA, Leon-Jacinto U, Aguilar-Vazquez A, Quirarte GL, Ramirez-Amaya V, Diaz-Cintra S. Increase of mushroom spine density in CA1 apical dendrites produced by water maze training is prevented by ovariectomy. Brain Res. 2011;1369:119–130. - PubMed
-
- Benshalom G. Determining the neuronal connectivity of Golgi-impregnated neurons: ultrastructural assessment of functional aspects. Microsc Res Tech. 1992;23:324–333. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

