Breadth of cellular and humoral immune responses elicited in rhesus monkeys by multi-valent mosaic and consensus immunogens
- PMID: 22521913
- PMCID: PMC3582221
- DOI: 10.1016/j.virol.2012.03.012
Breadth of cellular and humoral immune responses elicited in rhesus monkeys by multi-valent mosaic and consensus immunogens
Abstract
To create an HIV-1 vaccine that generates sufficient breadth of immune recognition to protect against the genetically diverse forms of the circulating virus, we have been exploring vaccines based on consensus and mosaic protein designs. Increasing the valency of a mosaic immunogen cocktail increases epitope coverage but with diminishing returns, as increasingly rare epitopes are incorporated into the mosaic proteins. In this study we compared the immunogenicity of 2-valent and 3-valent HIV-1 envelope mosaic immunogens in rhesus monkeys. Immunizations with the 3-valent mosaic immunogens resulted in a modest increase in the breadth of vaccine-elicited T lymphocyte responses compared to the 2-valent mosaic immunogens. However, the 3-valent mosaic immunogens elicited significantly higher neutralizing responses to Tier 1 viruses than the 2-valent mosaic immunogens. These findings underscore the potential utility of polyvalent mosaic immunogens for eliciting both cellular and humoral immune responses to HIV-1.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
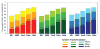
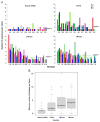
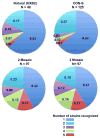
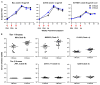
Similar articles
-
Rapid Induction of Multifunctional Antibodies in Rabbits and Macaques by Clade C HIV-1 CAP257 Envelopes Circulating During Epitope-Specific Neutralization Breadth Development.Front Immunol. 2020 Jun 2;11:984. doi: 10.3389/fimmu.2020.00984. eCollection 2020. Front Immunol. 2020. PMID: 32582155 Free PMC article.
-
Comparison of Immunogenicity in Rhesus Macaques of Transmitted-Founder, HIV-1 Group M Consensus, and Trivalent Mosaic Envelope Vaccines Formulated as a DNA Prime, NYVAC, and Envelope Protein Boost.J Virol. 2015 Jun;89(12):6462-80. doi: 10.1128/JVI.00383-15. Epub 2015 Apr 8. J Virol. 2015. PMID: 25855741 Free PMC article.
-
Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys.Nat Med. 2010 Mar;16(3):324-8. doi: 10.1038/nm.2108. Epub 2010 Feb 21. Nat Med. 2010. PMID: 20173754 Free PMC article.
-
Rational design of vaccines to elicit broadly neutralizing antibodies to HIV-1.Cold Spring Harb Perspect Med. 2011 Sep;1(1):a007278. doi: 10.1101/cshperspect.a007278. Cold Spring Harb Perspect Med. 2011. PMID: 22229123 Free PMC article. Review.
-
Stabilized HIV-1 envelope glycoprotein trimers for vaccine use.Curr Opin HIV AIDS. 2017 May;12(3):241-249. doi: 10.1097/COH.0000000000000363. Curr Opin HIV AIDS. 2017. PMID: 28422788 Free PMC article. Review.
Cited by
-
Distinct evolutionary pressures underlie diversity in simian immunodeficiency virus and human immunodeficiency virus lineages.J Virol. 2012 Dec;86(24):13217-31. doi: 10.1128/JVI.01862-12. Epub 2012 Oct 10. J Virol. 2012. PMID: 23055550 Free PMC article.
-
Induction of Potent and Long-Lived Antibody and Cellular Immune Responses in the Genitorectal Mucosa Could be the Critical Determinant of HIV Vaccine Efficacy.Front Immunol. 2014 May 8;5:202. doi: 10.3389/fimmu.2014.00202. eCollection 2014. Front Immunol. 2014. PMID: 24847327 Free PMC article. Review.
-
Polyvalent vaccine approaches to combat HIV-1 diversity.Immunol Rev. 2017 Jan;275(1):230-244. doi: 10.1111/imr.12516. Immunol Rev. 2017. PMID: 28133800 Free PMC article. Review.
-
Increased sequence coverage through combined targeting of variant and conserved epitopes correlates with control of HIV replication.J Virol. 2014 Jan;88(2):1354-65. doi: 10.1128/JVI.02361-13. Epub 2013 Nov 13. J Virol. 2014. PMID: 24227851 Free PMC article.
-
The influence of delivery vectors on HIV vaccine efficacy.Front Microbiol. 2014 Aug 22;5:439. doi: 10.3389/fmicb.2014.00439. eCollection 2014. Front Microbiol. 2014. PMID: 25202303 Free PMC article. Review.
References
-
- Barouch DH, O’Brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, Sun YH, La Porte A, Riggs AM, Lynch DM, Clark SL, Backus K, Perry JR, Seaman MS, Carville A, Mansfield KG, Szinger JJ, Fischer W, Muldoon M, Korber B. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med. 2010;16 (3):319–323. - PMC - PubMed
-
- Barouch DH, Yang ZY, Kong WP, Korioth-Schmitz B, Sumida SM, Truitt DM, Kishko MG, Arthur JC, Miura A, Mascola JR, Letvin NL, Nabel GJ. A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates. J Virol. 2005;79 (14):8828–8834. - PMC - PubMed
-
- Ferre AL, Lemongello D, Hunt PW, Morris MM, Garcia JC, Pollard RB, Yee HF, Jr, Martin JN, Deeks SG, Shacklett BL. Immunodominant HIV-specific CD8+ T-cell responses are common to blood and gastrointestinal mucosa, and Gag-specific responses dominate in rectal mucosa of HIV controllers. J Virol. 2010;84 (19):10354–10365. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

