Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics
- PMID: 22517853
- PMCID: PMC3357493
- DOI: 10.1126/science.1219192
Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics
Abstract
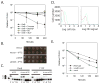
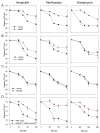
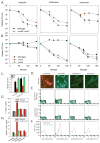
A detailed understanding of the mechanisms that underlie antibiotic killing is important for the derivation of new classes of antibiotics and clinically useful adjuvants for current antimicrobial therapies. Our efforts to understand why DinB (DNA polymerase IV) overproduction is cytotoxic to Escherichia coli led to the unexpected insight that oxidation of guanine to 8-oxo-guanine in the nucleotide pool underlies much of the cell death caused by both DinB overproduction and bactericidal antibiotics. We propose a model in which the cytotoxicity of beta-lactams and quinolones predominantly results from lethal double-strand DNA breaks caused by incomplete repair of closely spaced 8-oxo-deoxyguanosine lesions, whereas the cytotoxicity of aminoglycosides might additionally result from mistranslation due to the incorporation of 8-oxo-guanine into newly synthesized RNAs.
Figures

Similar articles
-
A novel mechanism for preventing mutations caused by oxidation of guanine nucleotides.EMBO Rep. 2003 May;4(5):479-83. doi: 10.1038/sj.embor.embor838. EMBO Rep. 2003. PMID: 12717453 Free PMC article.
-
Role of accessory DNA polymerases in DNA replication in Escherichia coli: analysis of the dnaX36 mutator mutant.J Bacteriol. 2008 Mar;190(5):1730-42. doi: 10.1128/JB.01463-07. Epub 2007 Dec 21. J Bacteriol. 2008. PMID: 18156258 Free PMC article.
-
Bactericidal Antibiotics Induce Toxic Metabolic Perturbations that Lead to Cellular Damage.Cell Rep. 2015 Nov 3;13(5):968-80. doi: 10.1016/j.celrep.2015.09.059. Epub 2015 Oct 22. Cell Rep. 2015. PMID: 26565910 Free PMC article.
-
Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases.Free Radic Biol Med. 2017 Jun;107:179-201. doi: 10.1016/j.freeradbiomed.2016.11.042. Epub 2016 Nov 27. Free Radic Biol Med. 2017. PMID: 27903453 Review.
-
Not breathing is not an option: How to deal with oxidative DNA damage.DNA Repair (Amst). 2017 Nov;59:82-105. doi: 10.1016/j.dnarep.2017.09.007. Epub 2017 Sep 22. DNA Repair (Amst). 2017. PMID: 28963982 Review.
Cited by
-
DNA polymerase θ specializes in incorporating synthetic expanded-size (xDNA) nucleotides.Nucleic Acids Res. 2016 Nov 2;44(19):9381-9392. doi: 10.1093/nar/gkw721. Epub 2016 Sep 2. Nucleic Acids Res. 2016. PMID: 27591252 Free PMC article.
-
Antibiotic treatment enhances the genome-wide mutation rate of target cells.Proc Natl Acad Sci U S A. 2016 May 3;113(18):E2498-505. doi: 10.1073/pnas.1601208113. Epub 2016 Apr 18. Proc Natl Acad Sci U S A. 2016. PMID: 27091991 Free PMC article.
-
Escherichia coli DNA polymerase IV (Pol IV), but not Pol II, dynamically switches with a stalled Pol III* replicase.J Bacteriol. 2012 Jul;194(14):3589-600. doi: 10.1128/JB.00520-12. Epub 2012 Apr 27. J Bacteriol. 2012. PMID: 22544274 Free PMC article.
-
Quantitative analysis of persister fractions suggests different mechanisms of formation among environmental isolates of E. coli.BMC Microbiol. 2013 Feb 4;13:25. doi: 10.1186/1471-2180-13-25. BMC Microbiol. 2013. PMID: 23379956 Free PMC article.
-
The C-Terminal Acid Phosphatase Module of the RNase HI Enzyme RnhC Controls Rifampin Sensitivity and Light-Dependent Colony Pigmentation of Mycobacterium smegmatis.J Bacteriol. 2023 Apr 25;205(4):e0043122. doi: 10.1128/jb.00431-22. Epub 2023 Mar 14. J Bacteriol. 2023. PMID: 36916909 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

