Connexin-43 in the osteogenic BM niche regulates its cellular composition and the bidirectional traffic of hematopoietic stem cells and progenitors
- PMID: 22498741
- PMCID: PMC3369607
- DOI: 10.1182/blood-2011-07-368506
Connexin-43 in the osteogenic BM niche regulates its cellular composition and the bidirectional traffic of hematopoietic stem cells and progenitors
Abstract
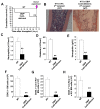
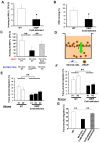
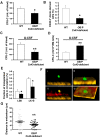
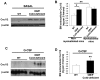
Connexin-43 (Cx43), a gap junction protein involved in control of cell proliferation, differentiation and migration, has been suggested to have a role in hematopoiesis. Cx43 is highly expressed in osteoblasts and osteogenic progenitors (OB/P). To elucidate the biologic function of Cx43 in the hematopoietic microenvironment (HM) and its influence in hematopoietic stem cell (HSC) activity, we studied the hematopoietic function in an in vivo model of constitutive deficiency of Cx43 in OB/P. The deficiency of Cx43 in OB/P cells does not impair the steady state hematopoiesis, but disrupts the directional trafficking of HSC/progenitors (Ps) between the bone marrow (BM) and peripheral blood (PB). OB/P Cx43 is a crucial positive regulator of transstromal migration and homing of both HSCs and progenitors in an irradiated microenvironment. However, OB/P Cx43 deficiency in nonmyeloablated animals does not result in a homing defect but induces increased endosteal lodging and decreased mobilization of HSC/Ps associated with proliferation and expansion of Cxcl12-secreting mesenchymal/osteolineage cells in the BM HM in vivo. Cx43 controls the cellular content of the BM osteogenic microenvironment and is required for homing of HSC/Ps in myeloablated animals.
Figures

Similar articles
-
Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells.Proc Natl Acad Sci U S A. 2012 Jun 5;109(23):9071-6. doi: 10.1073/pnas.1120358109. Epub 2012 May 18. Proc Natl Acad Sci U S A. 2012. PMID: 22611193 Free PMC article.
-
Bone marrow connexin-43 expression is critical for hematopoietic regeneration after chemotherapy.Cell Commun Adhes. 2005 Jul-Dec;12(5-6):307-17. doi: 10.1080/15419060500514200. Cell Commun Adhes. 2005. PMID: 16531325
-
Differential regulation of CXCL5 by FGF2 in osteoblastic and endothelial niche cells supports hematopoietic stem cell migration.Stem Cells Dev. 2012 Dec 10;21(18):3391-402. doi: 10.1089/scd.2012.0128. Epub 2012 Sep 4. Stem Cells Dev. 2012. PMID: 22827607 Free PMC article.
-
Gap Junctions in the Bone Marrow Lympho-Hematopoietic Stem Cell Niche, Leukemia Progression, and Chemoresistance.Int J Mol Sci. 2020 Jan 25;21(3):796. doi: 10.3390/ijms21030796. Int J Mol Sci. 2020. PMID: 31991829 Free PMC article. Review.
-
Analyzing hematopoietic stem cell homing, lodgment, and engraftment to better understand the bone marrow niche.Ann N Y Acad Sci. 2014 Mar;1310:119-28. doi: 10.1111/nyas.12329. Epub 2014 Jan 15. Ann N Y Acad Sci. 2014. PMID: 24428368 Review.
Cited by
-
Connexins and Pannexins in Bone and Skeletal Muscle.Curr Osteoporos Rep. 2017 Aug;15(4):326-334. doi: 10.1007/s11914-017-0374-z. Curr Osteoporos Rep. 2017. PMID: 28647887 Free PMC article. Review.
-
Deficiency of GRP94 in the hematopoietic system alters proliferation regulators in hematopoietic stem cells.Stem Cells Dev. 2013 Dec 1;22(23):3062-73. doi: 10.1089/scd.2013.0181. Epub 2013 Aug 20. Stem Cells Dev. 2013. PMID: 23859598 Free PMC article.
-
Innate immunity orchestrates the mobilization and homing of hematopoietic stem/progenitor cells by engaging purinergic signaling-an update.Purinergic Signal. 2020 Jun;16(2):153-166. doi: 10.1007/s11302-020-09698-y. Epub 2020 May 15. Purinergic Signal. 2020. PMID: 32415576 Free PMC article. Review.
-
Defective cancellous bone structure and abnormal response to PTH in cortical bone of mice lacking Cx43 cytoplasmic C-terminus domain.Bone. 2015 Dec;81:632-643. doi: 10.1016/j.bone.2015.09.011. Epub 2015 Sep 26. Bone. 2015. PMID: 26409319 Free PMC article.
-
Molecular mechanisms of osteoblast/osteocyte regulation by connexin43.Calcif Tissue Int. 2014 Jan;94(1):55-67. doi: 10.1007/s00223-013-9742-6. Epub 2013 Jun 11. Calcif Tissue Int. 2014. PMID: 23754488 Free PMC article. Review.
References
-
- Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. - PubMed
-
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. - PubMed
-
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–988. - PubMed
-
- Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

