Functions of the Epstein-Barr virus EBNA1 protein in viral reactivation and lytic infection
- PMID: 22491455
- PMCID: PMC3372196
- DOI: 10.1128/JVI.00013-12
Functions of the Epstein-Barr virus EBNA1 protein in viral reactivation and lytic infection
Abstract
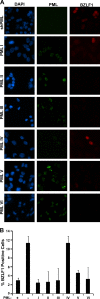
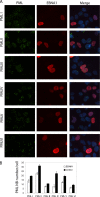
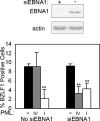
EBNA1 is the only nuclear Epstein-Barr virus (EBV) protein expressed in both latent and lytic modes of infection. While EBNA1 is known to play several important roles in latent infection, the reason for its continued expression in lytic infection is unknown. Here we identified two roles for EBNA1 in the reactivation of latent EBV to the lytic cycle in epithelial cells. First, EBNA1 depletion in latently infected cells was shown to positively contribute to spontaneous EBV reactivation, showing that EBNA1 has a role in suppressing reactivation. Second, when the lytic cycle was induced, EBNA1 depletion decreased lytic gene expression and DNA amplification, showing that it positively contributed to lytic infection. Since we have previously shown that EBNA1 disrupts promyelocytic leukemia (PML) nuclear bodies, we investigated whether this function could account for the effects of EBNA1 on lytic infection by repeating the experiments with cells lacking PML proteins. In the absence of PML, EBNA1 did not promote lytic infection, indicating that the EBNA1-mediated PML disruption is responsible for promoting lytic infection. In keeping with this conclusion, PML silencing was found to be sufficient to induce the EBV lytic cycle. Finally, by generating cells with single PML isoforms, we showed that individual PML isoforms were sufficient to suppress EBV lytic reactivation, although PML isoform IV (PML IV) was ineffective because it was most efficiently degraded by EBNA1. Our results provide the first function for EBNA1 in lytic infection and show that EBNA1 interactions with PML IV lead to a loss of PML nuclear bodies (NBs) that promotes lytic infection.
Figures

Similar articles
-
Contributions of the Epstein-Barr virus EBNA1 protein to gastric carcinoma.J Virol. 2012 Jan;86(1):60-8. doi: 10.1128/JVI.05623-11. Epub 2011 Oct 19. J Virol. 2012. PMID: 22013060 Free PMC article.
-
Epstein-Barr nuclear antigen 1 contributes to nasopharyngeal carcinoma through disruption of PML nuclear bodies.PLoS Pathog. 2008 Oct 3;4(10):e1000170. doi: 10.1371/journal.ppat.1000170. PLoS Pathog. 2008. PMID: 18833293 Free PMC article.
-
Viral disruption of promyelocytic leukemia (PML) nuclear bodies by hijacking host PML regulators.Virulence. 2011 Jan-Feb;2(1):58-62. doi: 10.4161/viru.2.1.14610. Epub 2011 Jan 1. Virulence. 2011. PMID: 21217204
-
Contributions of Epstein-Barr nuclear antigen 1 (EBNA1) to cell immortalization and survival.Viruses. 2012 Sep;4(9):1537-1547. doi: 10.3390/v4091537. Epub 2012 Sep 13. Viruses. 2012. PMID: 23170171 Free PMC article. Review.
-
EBNA1.Curr Top Microbiol Immunol. 2015;391:3-34. doi: 10.1007/978-3-319-22834-1_1. Curr Top Microbiol Immunol. 2015. PMID: 26428370 Review.
Cited by
-
The Role of Nuclear Antiviral Factors against Invading DNA Viruses: The Immediate Fate of Incoming Viral Genomes.Viruses. 2016 Oct 22;8(10):290. doi: 10.3390/v8100290. Viruses. 2016. PMID: 27782081 Free PMC article. Review.
-
A role for the nucleosome assembly proteins TAF-Iβ and NAP1 in the activation of BZLF1 expression and Epstein-Barr virus reactivation.PLoS One. 2013 May 14;8(5):e63802. doi: 10.1371/journal.pone.0063802. Print 2013. PLoS One. 2013. PMID: 23691099 Free PMC article.
-
Chromosomal fragile site breakage by EBV-encoded EBNA1 at clustered repeats.Nature. 2023 Apr;616(7957):504-509. doi: 10.1038/s41586-023-05923-x. Epub 2023 Apr 12. Nature. 2023. PMID: 37046091 Free PMC article.
-
Role of Epstein-Barr Virus and Human Papillomavirus Coinfection in Cervical Cancer: Epidemiology, Mechanisms and Perspectives.Pathogens. 2020 Aug 21;9(9):685. doi: 10.3390/pathogens9090685. Pathogens. 2020. PMID: 32839399 Free PMC article. Review.
-
The interplay between Epstein-Bar virus (EBV) with the p53 and its homologs during EBV associated malignancies.Heliyon. 2019 Nov 14;5(11):e02624. doi: 10.1016/j.heliyon.2019.e02624. eCollection 2019 Nov. Heliyon. 2019. PMID: 31840114 Free PMC article. Review.
References
-
- Ahn JH, Hayward GS. 2000. Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection. Virology 274:39–55 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

