NLRC4-driven production of IL-1β discriminates between pathogenic and commensal bacteria and promotes host intestinal defense
- PMID: 22484733
- PMCID: PMC3361590
- DOI: 10.1038/ni.2263
NLRC4-driven production of IL-1β discriminates between pathogenic and commensal bacteria and promotes host intestinal defense
Abstract
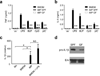
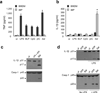
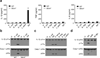
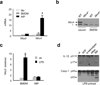
Intestinal phagocytes transport oral antigens and promote immune tolerance, but their role in innate immune responses remains unclear. Here we found that intestinal phagocytes were anergic to ligands for Toll-like receptors (TLRs) or commensals but constitutively expressed the precursor to interleukin 1β (pro-IL-1β). After infection with pathogenic Salmonella or Pseudomonas, intestinal phagocytes produced mature IL-1β through the NLRC4 inflammasome but did not produce tumor necrosis factor (TNF) or IL-6. BALB/c mice deficient in NLRC4 or the IL-1 receptor were highly susceptible to orogastric but not intraperitoneal infection with Salmonella. That enhanced lethality was preceded by impaired expression of endothelial adhesion molecules, lower neutrophil recruitment and poor intestinal pathogen clearance. Thus, NLRC4-dependent production of IL-1β by intestinal phagocytes represents a specific response that discriminates pathogenic bacteria from commensal bacteria and contributes to host defense in the intestine.
Figures



Comment in
-
For gut's sake: NLRC4 inflammasomes distinguish friend from foe.Nat Immunol. 2012 Apr 18;13(5):429-31. doi: 10.1038/ni.2289. Nat Immunol. 2012. PMID: 22513324 No abstract available.
Similar articles
-
Bacterial Outer Membrane Vesicle-Mediated Cytosolic Delivery of Flagellin Triggers Host NLRC4 Canonical Inflammasome Signaling.Front Immunol. 2020 Nov 18;11:581165. doi: 10.3389/fimmu.2020.581165. eCollection 2020. Front Immunol. 2020. PMID: 33312172 Free PMC article.
-
For gut's sake: NLRC4 inflammasomes distinguish friend from foe.Nat Immunol. 2012 Apr 18;13(5):429-31. doi: 10.1038/ni.2289. Nat Immunol. 2012. PMID: 22513324 No abstract available.
-
The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge.Cell Rep. 2014 Jul 24;8(2):570-82. doi: 10.1016/j.celrep.2014.06.028. Epub 2014 Jul 17. Cell Rep. 2014. PMID: 25043180
-
Advances in Understanding Activation and Function of the NLRC4 Inflammasome.Int J Mol Sci. 2021 Jan 21;22(3):1048. doi: 10.3390/ijms22031048. Int J Mol Sci. 2021. PMID: 33494299 Free PMC article. Review.
-
Nlrc4/Ipaf/CLAN/CARD12: more than a flagellin sensor.Int J Biochem Cell Biol. 2010 Jun;42(6):789-91. doi: 10.1016/j.biocel.2010.01.003. Epub 2010 Jan 11. Int J Biochem Cell Biol. 2010. PMID: 20067841 Free PMC article. Review.
Cited by
-
Reciprocal interactions of the intestinal microbiota and immune system.Nature. 2012 Sep 13;489(7415):231-41. doi: 10.1038/nature11551. Nature. 2012. PMID: 22972296 Free PMC article. Review.
-
Of inflammasomes and pathogens--sensing of microbes by the inflammasome.EMBO Mol Med. 2013 Jun;5(6):814-26. doi: 10.1002/emmm.201201771. Epub 2013 May 13. EMBO Mol Med. 2013. PMID: 23666718 Free PMC article. Review.
-
Artificial parasin I protein (API) supplementation improves growth performance and intestinal health in weaned piglets challenged with enterotoxigenic Escherichia coli.Anim Nutr. 2024 May 22;18:154-165. doi: 10.1016/j.aninu.2024.04.015. eCollection 2024 Sep. Anim Nutr. 2024. PMID: 39263444 Free PMC article.
-
Vibrio parahaemolyticus effector proteins suppress inflammasome activation by interfering with host autophagy signaling.PLoS Pathog. 2013 Jan;9(1):e1003142. doi: 10.1371/journal.ppat.1003142. Epub 2013 Jan 24. PLoS Pathog. 2013. PMID: 23357873 Free PMC article.
-
Regulation of inflammasome activation.Immunol Rev. 2015 May;265(1):6-21. doi: 10.1111/imr.12296. Immunol Rev. 2015. PMID: 25879280 Free PMC article. Review.
References
-
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. - PubMed
-
- Franchi L, Munoz-Planillo R, Reimer T, Eigenbrod T, Nunez G. Inflammasomes as microbial sensors. Eur J Immunol. 2010;40:611–615. - PubMed
-
- Franchi L, et al. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10:1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK091191-08/DK/NIDDK NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- R01 DK61707/DK/NIDDK NIH HHS/United States
- T32 HL007517/HL/NHLBI NIH HHS/United States
- R01 DK061707/DK/NIDDK NIH HHS/United States
- R01 AI063331/AI/NIAID NIH HHS/United States
- R01 DK091191/DK/NIDDK NIH HHS/United States
- CA46952/CA/NCI NIH HHS/United States
- 5 P30 CA46592/CA/NCI NIH HHS/United States
- T32 HD007505/HD/NICHD NIH HHS/United States
- R01 AI063331-08/AI/NIAID NIH HHS/United States
- T32-HL007517/HL/NHLBI NIH HHS/United States
- R01 AI063331-07/AI/NIAID NIH HHS/United States
- R01 DK091191-07/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

