Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish
- PMID: 22482805
- PMCID: PMC3322236
- DOI: 10.1016/j.ajhg.2012.02.026
Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish
Abstract
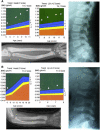
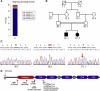
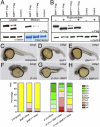
Bone morphogenetic protein 1 (BMP1) is an astacin metalloprotease with important cellular functions and diverse substrates, including extracellular-matrix proteins and antagonists of some TGFβ superfamily members. Combining whole-exome sequencing and filtering for homozygous stretches of identified variants, we found a homozygous causative BMP1 mutation, c.34G>C, in a consanguineous family affected by increased bone mineral density and multiple recurrent fractures. The mutation is located within the BMP1 signal peptide and leads to impaired secretion and an alteration in posttranslational modification. We also characterize a zebrafish bone mutant harboring lesions in bmp1a, demonstrating conservation of BMP1 function in osteogenesis across species. Genetic, biochemical, and histological analyses of this mutant and a comparison to a second, similar locus reveal that Bmp1a is critically required for mature-collagen generation, downstream of osteoblast maturation, in bone. We thus define the molecular and cellular bases of BMP1-dependent osteogenesis and show the importance of this protein for bone formation and stability.
Copyright © 2012 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Bone morphogenetic protein (BMP)1-3 enhances bone repair.Biochem Biophys Res Commun. 2011 Apr 29;408(1):25-31. doi: 10.1016/j.bbrc.2011.03.109. Epub 2011 Mar 29. Biochem Biophys Res Commun. 2011. PMID: 21453682
-
Report of a newly indentified patient with mutations in BMP1 and underlying pathogenetic aspects.Am J Med Genet A. 2014 May;164A(5):1143-50. doi: 10.1002/ajmg.a.36427. Epub 2014 Mar 19. Am J Med Genet A. 2014. PMID: 24648371
-
Phenotypic variability in patients with osteogenesis imperfecta caused by BMP1 mutations.Am J Med Genet A. 2016 Dec;170(12):3150-3156. doi: 10.1002/ajmg.a.37958. Epub 2016 Aug 30. Am J Med Genet A. 2016. PMID: 27576954
-
[Diagnosis and treatment of immobilization osteoporosis].Nihon Rinsho. 2007 Nov 28;65 Suppl 9:514-9. Nihon Rinsho. 2007. PMID: 18159714 Review. Japanese. No abstract available.
-
[Role of bone morphogenetic protein 1/tolloid proteinase family in the development of teeth and bone].Hua Xi Kou Qiang Yi Xue Za Zhi. 2020 Oct 1;38(5):589-593. doi: 10.7518/hxkq.2020.05.020. Hua Xi Kou Qiang Yi Xue Za Zhi. 2020. PMID: 33085247 Free PMC article. Review. Chinese.
Cited by
-
Bone collagen: new clues to its mineralization mechanism from recessive osteogenesis imperfecta.Calcif Tissue Int. 2013 Oct;93(4):338-47. doi: 10.1007/s00223-013-9723-9. Epub 2013 Mar 19. Calcif Tissue Int. 2013. PMID: 23508630 Free PMC article. Review.
-
Can Bone Morphogenetic Protein 1 (BMP1) Be a Potential Biomarker of Obesity?Cureus. 2024 Aug 16;16(8):e67025. doi: 10.7759/cureus.67025. eCollection 2024 Aug. Cureus. 2024. PMID: 39280566 Free PMC article.
-
Mutation of foxl1 Results in Reduced Cartilage Markers in a Zebrafish Model of Otosclerosis.Genes (Basel). 2022 Jun 21;13(7):1107. doi: 10.3390/genes13071107. Genes (Basel). 2022. PMID: 35885890 Free PMC article.
-
Phenomics-Based Quantification of CRISPR-Induced Mosaicism in Zebrafish.Cell Syst. 2020 Mar 25;10(3):275-286.e5. doi: 10.1016/j.cels.2020.02.007. Epub 2020 Mar 18. Cell Syst. 2020. PMID: 32191876 Free PMC article.
-
Proteomic Analysis of Mesenchymal Stromal Cells Secretome in Comparison to Leukocyte- and Platelet-Rich Fibrin.Int J Mol Sci. 2023 Aug 22;24(17):13057. doi: 10.3390/ijms241713057. Int J Mol Sci. 2023. PMID: 37685865 Free PMC article.
References
-
- Byers P.H., Cole W.G. Osteogenesis Imperfecta. In: Royce P., Steinmann B., editors. Connective Tissue and its Heritable Disorders: Molecular, Genetic, and Medical Aspects. Second Edition. John Wiley & Sons; Hoboken, NJ: 2002. pp. 385–430.
-
- Sillence D.O., Rimoin D.L. Classification of osteogenesis imperfect. Lancet. 1978;1:1041–1042. - PubMed
-
- Basel D., Steiner R.D. Osteogenesis imperfecta: Recent findings shed new light on this once well-understood condition. Genet. Med. 2009;11:375–385. - PubMed
-
- Rauch F., Glorieux F.H. Osteogenesis imperfecta. Lancet. 2004;363:1377–1385. - PubMed
-
- Marini J.C., Forlino A., Cabral W.A., Barnes A.M., San Antonio J.D., Milgrom S., Hyland J.C., Körkkö J., Prockop D.J., De Paepe A. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: Regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum. Mutat. 2007;28:209–221. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC2 HL102926/HL/NHLBI NIH HHS/United States
- 01GM0880/GM/NIGMS NIH HHS/United States
- HL-102924/HL/NHLBI NIH HHS/United States
- RC2 HL102924/HL/NHLBI NIH HHS/United States
- HL-102926/HL/NHLBI NIH HHS/United States
- RC2 HL103010/HL/NHLBI NIH HHS/United States
- HL-102923/HL/NHLBI NIH HHS/United States
- RC2 HL102923/HL/NHLBI NIH HHS/United States
- UC2 HL102926/HL/NHLBI NIH HHS/United States
- UC2 HL103010/HL/NHLBI NIH HHS/United States
- HL-103010/HL/NHLBI NIH HHS/United States
- UC2 HL102923/HL/NHLBI NIH HHS/United States
- UC2 HL102924/HL/NHLBI NIH HHS/United States
- R01 AR047746/AR/NIAMS NIH HHS/United States
- HL-102925/HL/NHLBI NIH HHS/United States
- RC2 HL102925/HL/NHLBI NIH HHS/United States
- UC2 HL102925/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

