The interaction of Hsc70 protein with fibrillar α-Synuclein and its therapeutic potential in Parkinson's disease
- PMID: 22482021
- PMCID: PMC3291325
- DOI: 10.4161/cib.18483
The interaction of Hsc70 protein with fibrillar α-Synuclein and its therapeutic potential in Parkinson's disease
Abstract
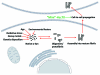
We recently described the effect of the constitutively expressed chaperone, Hsc70 protein, on α‑Synuclein aggregation, a phenomenon associated with Parkinson disease. In vitro, Hsc70 binds to soluble α‑Syn and slows down its assembly into fibrils. Hsc70 also binds fibrillar α‑Syn, 5-fold tighter than soluble α‑Syn. This interaction reduces the cytotoxicity associated with naked α‑Syn fibrils. Herein, we discuss the feasibility of engineering a "minichaperone" which could be used against α‑Syn assembly propagation in Parkinson disease: taking what is necessary and sufficient within Hsc70 to protect against the damaging repercussions of high molecular weight α‑Syn species' passage from one neuron to another in the brain.
Keywords: Hsc70; Parkinson’s disease; alpha synuclein; chaperones; heat shock protein.
Figures
Comment on
- Pemberton S, Madiona K, Pieri L, Kabani M, Bousset L, Melki R. Hsc70 Protein Interaction with Soluble and Fibrillar α‑Synuclein. J Biol Chem. 2011;286:34690–9. doi: 10.1074/jbc.M111.261321.
Similar articles
-
Identification of protein interfaces between α-synuclein, the principal component of Lewy bodies in Parkinson disease, and the molecular chaperones human Hsc70 and the yeast Ssa1p.J Biol Chem. 2012 Sep 21;287(39):32630-9. doi: 10.1074/jbc.M112.387530. Epub 2012 Jul 26. J Biol Chem. 2012. PMID: 22843682 Free PMC article.
-
Polypeptides derived from α-Synuclein binding partners to prevent α-Synuclein fibrils interaction with and take-up by cells.PLoS One. 2020 Aug 13;15(8):e0237328. doi: 10.1371/journal.pone.0237328. eCollection 2020. PLoS One. 2020. PMID: 32790707 Free PMC article.
-
Hsc70 protein interaction with soluble and fibrillar alpha-synuclein.J Biol Chem. 2011 Oct 7;286(40):34690-9. doi: 10.1074/jbc.M111.261321. Epub 2011 Aug 10. J Biol Chem. 2011. PMID: 21832061 Free PMC article.
-
The emerging role of α-synuclein truncation in aggregation and disease.J Biol Chem. 2020 Jul 24;295(30):10224-10244. doi: 10.1074/jbc.REV120.011743. Epub 2020 May 18. J Biol Chem. 2020. PMID: 32424039 Free PMC article. Review.
-
Advances in modelling alpha-synuclein-induced Parkinson's diseases in rodents: Virus-based models versus inoculation of exogenous preformed toxic species.J Neurosci Methods. 2020 May 15;338:108685. doi: 10.1016/j.jneumeth.2020.108685. Epub 2020 Mar 12. J Neurosci Methods. 2020. PMID: 32173400 Review.
Cited by
-
Protein Partners of α-Synuclein in Health and Disease.Brain Pathol. 2016 May;26(3):389-97. doi: 10.1111/bpa.12374. Brain Pathol. 2016. PMID: 26940507 Free PMC article. Review.
-
Alpha-synuclein, Proteotoxicity and Parkinson's Disease: Search for Neuroprotective Therapy.Curr Neuropharmacol. 2018;16(7):1086-1097. doi: 10.2174/1570159X15666171129100944. Curr Neuropharmacol. 2018. PMID: 29189163 Free PMC article. Review.
-
Hsc70 chaperone activity underlies Trio GEF function in axon growth and guidance induced by netrin-1.J Cell Biol. 2015 Aug 31;210(5):817-32. doi: 10.1083/jcb.201505084. J Cell Biol. 2015. PMID: 26323693 Free PMC article.
-
Identification of protein interfaces between α-synuclein, the principal component of Lewy bodies in Parkinson disease, and the molecular chaperones human Hsc70 and the yeast Ssa1p.J Biol Chem. 2012 Sep 21;287(39):32630-9. doi: 10.1074/jbc.M112.387530. Epub 2012 Jul 26. J Biol Chem. 2012. PMID: 22843682 Free PMC article.
-
Polypeptides derived from α-Synuclein binding partners to prevent α-Synuclein fibrils interaction with and take-up by cells.PLoS One. 2020 Aug 13;15(8):e0237328. doi: 10.1371/journal.pone.0237328. eCollection 2020. PLoS One. 2020. PMID: 32790707 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous