Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy
- PMID: 22481232
- PMCID: PMC3465940
- DOI: 10.1097/JTO.0b013e318247504a
Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy
Abstract
Introduction: We evaluated the ability of histopathologic response criteria to predict overall survival (OS) and disease-free survival (DFS) in patients with surgically resected non-small cell lung cancer (NSCLC) treated with or without neoadjuvant chemotherapy.
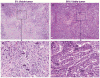
Methods: Tissue specimens from 358 patients with NSCLC were evaluated by pathologists blinded to the patient treatment and outcome. The surgical specimens were reviewed for various histopathologic features in the tumor including percentage of residual viable tumor cells, necrosis, and fibrosis. The relationship between the histopathologic findings and OS was assessed.
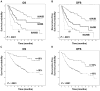
Results: The percentage of residual viable tumor cells and surgical pathologic stage were associated with OS and DFS in 192 patients with NSCLC receiving neoadjuvant chemotherapy in multivariate analysis (p = 0.005 and p = 0.01, respectively). There was no association of OS or DFS with percentage of viable tumor cells in 166 patients with NSCLC who did not receive neoadjuvant chemotherapy (p = 0.31 and p = 0.45, respectively). Long-term OS and DFS were significantly prolonged in patients who had ≤10% viable tumor compared with patients with >10% viable tumor cells (5 years OS, 85% versus 40%, p < 0.0001 and 5 years DFS, 78% versus 35%, p < 0.001).
Conclusion: The percentages of residual viable tumor cells predict OS and DFS in patients with resected NSCLC after neoadjuvant chemotherapy even when controlled for pathologic stage. Histopathologic assessment of resected specimens after neoadjuvant chemotherapy could potentially have a role in addition to pathologic stage in assessing prognosis, chemotherapy response, and the need for additional adjuvant therapies.
Conflict of interest statement
Disclosure: The authors declare no conflicts of interest.
Figures

Similar articles
-
Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy.J Thorac Oncol. 2013 Feb;8(2):222-8. doi: 10.1097/JTO.0b013e3182774108. J Thorac Oncol. 2013. PMID: 23287849 Free PMC article.
-
Survival analysis in advanced non small cell lung cancer treated with platinum based chemotherapy in combination with paclitaxel, gemcitabine and etoposide.Asian Pac J Cancer Prev. 2013;14(8):4661-6. doi: 10.7314/apjcp.2013.14.8.4661. Asian Pac J Cancer Prev. 2013. PMID: 24083721
-
A retrospective study: platinum-based induction chemotherapy combined with gemcitabine or paclitaxel for stage IIB-IIIA central non-small-cell lung cancer.World J Surg Oncol. 2013 Mar 21;11:76. doi: 10.1186/1477-7819-11-76. World J Surg Oncol. 2013. PMID: 23517534 Free PMC article.
-
Neoadjuvant chemotherapy followed by surgery in lung cancer: Indian scenario.Curr Probl Cancer. 2020 Jun;44(3):100563. doi: 10.1016/j.currproblcancer.2020.100563. Epub 2020 Mar 4. Curr Probl Cancer. 2020. PMID: 32265058 Review.
-
Neoadjuvant strategies for non-small cell lung cancer.Suppl Tumori. 2004 Mar-Apr;3(2):S49-51. Suppl Tumori. 2004. PMID: 15067968 Review. No abstract available.
Cited by
-
[Application of Neoadjuvant Immuno-chemotherapy in NSCLC].Zhongguo Fei Ai Za Zhi. 2021 Apr 20;24(4):284-292. doi: 10.3779/j.issn.1009-3419.2021.102.10. Zhongguo Fei Ai Za Zhi. 2021. PMID: 33910277 Free PMC article. Review. Chinese.
-
Neoadjuvant immunotherapy combined with chemotherapy in the treatment of stage III lung squamous cell carcinoma: a retrospective cohort study.J Thorac Dis. 2023 Oct 31;15(10):5658-5668. doi: 10.21037/jtd-23-1175. Epub 2023 Sep 12. J Thorac Dis. 2023. PMID: 37969291 Free PMC article.
-
Review and perspective on adjuvant and neoadjuvant immunotherapies in NSCLC.Onco Targets Ther. 2019 Sep 6;12:7329-7336. doi: 10.2147/OTT.S218321. eCollection 2019. Onco Targets Ther. 2019. PMID: 31564915 Free PMC article.
-
Progress on neoadjuvant immunotherapy in resectable non-small cell lung cancer and potential biomarkers.Front Oncol. 2023 Jan 24;12:1099304. doi: 10.3389/fonc.2022.1099304. eCollection 2022. Front Oncol. 2023. PMID: 36761426 Free PMC article. Review.
-
Predicting pathologic response to neoadjuvant chemoradiation in resectable stage III non-small cell lung cancer patients using computed tomography radiomic features.Lung Cancer. 2019 Sep;135:1-9. doi: 10.1016/j.lungcan.2019.06.020. Epub 2019 Jul 5. Lung Cancer. 2019. PMID: 31446979 Free PMC article.
References
-
- American Cancer Society. Cancer Facts and Figures. Atlanta, GA: American Cancer Society; 2008. [Accessed April 1, 2011.]. Available at: http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf.
-
- Roth JA, Atkinson EN, Fossella F, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer. 1998;21:1–6. - PubMed
-
- Rosell R, Gomez-Codina J, Camps C, et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized clinical trial. Lung Cancer. 1999;26:7–14. - PubMed
-
- Pisters KM, Ginsberg RJ, Giroux DJ, et al. Induction chemotherapy before surgery for early-stage lung cancer: a novel approach. Bimodality Lung Oncology Team. J Thorac Cardiovasc Surg. 2000;119:429–439. - PubMed
-
- Depierre A, Milleron B, Moro-Sibilot D, et al. Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIa non-small-cell lung cancer. J Clin Oncol. 2002;20:247–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

