Reductively responsive siRNA-conjugated hydrogel nanoparticles for gene silencing
- PMID: 22475061
- PMCID: PMC3357068
- DOI: 10.1021/ja300174v
Reductively responsive siRNA-conjugated hydrogel nanoparticles for gene silencing
Abstract
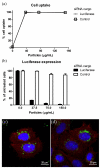
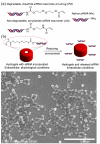
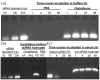
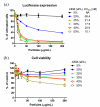
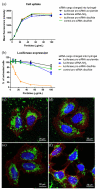
A critical need still remains for effective delivery of RNA interference (RNAi) therapeutics to target tissues and cells. Self-assembled lipid- and polymer-based systems have been most extensively explored for transfection with small interfering RNA (siRNA) in liver and cancer therapies. Safety and compatibility of materials implemented in delivery systems must be ensured to maximize therapeutic indices. Hydrogel nanoparticles of defined dimensions and compositions, prepared via a particle molding process that is a unique off-shoot of soft lithography known as particle replication in nonwetting templates (PRINT), were explored in these studies as delivery vectors. Initially, siRNA was encapsulated in particles through electrostatic association and physical entrapment. Dose-dependent gene silencing was elicited by PEGylated hydrogels at low siRNA doses without cytotoxicity. To prevent disassociation of cargo from particles after systemic administration or during postfabrication processing for surface functionalization, a polymerizable siRNA pro-drug conjugate with a degradable, disulfide linkage was prepared. Triggered release of siRNA from the pro-drug hydrogels was observed under a reducing environment while cargo retention and integrity were maintained under physiological conditions. Gene silencing efficiency and cytocompatibility were optimized by screening the amine content of the particles. When appropriate control siRNA cargos were loaded into hydrogels, gene knockdown was only encountered for hydrogels containing releasable, target-specific siRNAs, accompanied by minimal cell death. Further investigation into shape, size, and surface decoration of siRNA-conjugated hydrogels should enable efficacious targeted in vivo RNAi therapies.
© 2012 American Chemical Society
Figures

Similar articles
-
Reductively Responsive Hydrogel Nanoparticles with Uniform Size, Shape, and Tunable Composition for Systemic siRNA Delivery in Vivo.Mol Pharm. 2015 Oct 5;12(10):3518-3526. doi: 10.1021/acs.molpharmaceut.5b00054. Epub 2015 Sep 4. Mol Pharm. 2015. PMID: 26287725 Free PMC article.
-
Degradable poly(ethylene glycol) (PEG)-based hydrogels for spatiotemporal control of siRNA/nanoparticle delivery.J Control Release. 2018 Oct 10;287:58-66. doi: 10.1016/j.jconrel.2018.08.002. Epub 2018 Aug 3. J Control Release. 2018. PMID: 30077736 Free PMC article.
-
Controlled and sustained delivery of siRNA/NPs from hydrogels expedites bone fracture healing.Biomaterials. 2017 Sep;139:127-138. doi: 10.1016/j.biomaterials.2017.06.001. Epub 2017 Jun 4. Biomaterials. 2017. PMID: 28601703 Free PMC article.
-
High-density lipoproteins for the systemic delivery of short interfering RNA.Expert Opin Drug Deliv. 2014 Feb;11(2):231-47. doi: 10.1517/17425247.2014.866089. Epub 2013 Dec 9. Expert Opin Drug Deliv. 2014. PMID: 24313310 Free PMC article. Review.
-
Bioinspired Spatiotemporal Management toward RNA Therapies.ACS Nano. 2023 Dec 26;17(24):24539-24563. doi: 10.1021/acsnano.3c08219. Epub 2023 Dec 13. ACS Nano. 2023. PMID: 38091941 Review.
Cited by
-
Catalytic self-assembly of a DNA dendritic complex for efficient gene silencing.Chem Commun (Camb). 2016 Jan 25;52(7):1413-5. doi: 10.1039/c5cc06937h. Chem Commun (Camb). 2016. PMID: 26626818 Free PMC article.
-
Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management.Pharmaceutics. 2021 Mar 8;13(3):357. doi: 10.3390/pharmaceutics13030357. Pharmaceutics. 2021. PMID: 33800402 Free PMC article. Review.
-
Engineered PRINT(®) nanoparticles for controlled delivery of antigens and immunostimulants.Hum Vaccin Immunother. 2014;10(7):1908-13. doi: 10.4161/hv.28817. Hum Vaccin Immunother. 2014. PMID: 25424798 Free PMC article.
-
Analysis of the murine immune response to pulmonary delivery of precisely fabricated nano- and microscale particles.PLoS One. 2013 Apr 12;8(4):e62115. doi: 10.1371/journal.pone.0062115. Print 2013. PLoS One. 2013. PMID: 23593509 Free PMC article.
-
Fast-Forming Dissolvable Redox-Responsive Hydrogels: Exploiting the Orthogonality of Thiol-Maleimide and Thiol-Disulfide Exchange Chemistry.Biomacromolecules. 2022 Sep 12;23(9):3525-3534. doi: 10.1021/acs.biomac.2c00209. Epub 2022 Jun 13. Biomacromolecules. 2022. PMID: 35696518 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

