Fbw7 and p53 cooperatively suppress advanced and chromosomally unstable intestinal cancer
- PMID: 22473991
- PMCID: PMC3372235
- DOI: 10.1128/MCB.00305-12
Fbw7 and p53 cooperatively suppress advanced and chromosomally unstable intestinal cancer
Abstract
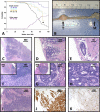
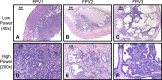
Colorectal cancer (CRC) remains a major cause of cancer mortality worldwide. Murine models have yielded critical insights into CRC pathogenesis, but they often fail to recapitulate advanced-disease phenotypes, notably metastasis and chromosomal instability (CIN). New models are thus needed to understand disease progression and to develop therapies. We sought to model advanced CRC by inactivating two tumor suppressors that are mutated in human CRCs, the Fbw7 ubiquitin ligase and p53. Here we report that Fbw7 deletion alters differentiation and proliferation in the gut epithelium and stabilizes oncogenic Fbw7 substrates, such as cyclin E and Myc. However, Fbw7 deletion does not cause tumorigenesis in the gut. In contrast, codeletion of both Fbw7 and p53 causes highly penetrant, aggressive, and metastatic adenocarcinomas, and allografts derived from these tumors form highly malignant adenocarcinomas. In vitro evidence indicates that Fbw7 ablation promotes genetic instability that is suppressed by p53, and we show that most Fbw7⁻/⁻; p53⁻/⁻ carcinomas exhibit a CIN⁺ phenotype. We conclude that Fbw7 and p53 synergistically suppress adenocarcinomas that mimic advanced human CRC with respect to histopathology, metastasis, and CIN. This model thus represents a novel tool for studies of advanced CRC as well as carcinogenesis associated with ubiquitin pathway mutations.
Figures


Similar articles
-
FBW7 mutations mediate resistance of colorectal cancer to targeted therapies by blocking Mcl-1 degradation.Oncogene. 2017 Feb 9;36(6):787-796. doi: 10.1038/onc.2016.247. Epub 2016 Jul 11. Oncogene. 2017. PMID: 27399335 Free PMC article.
-
F-box and WD repeat domain-containing 7 regulates intestinal cell lineage commitment and is a haploinsufficient tumor suppressor.Gastroenterology. 2010 Sep;139(3):929-41. doi: 10.1053/j.gastro.2010.05.078. Epub 2010 Jun 2. Gastroenterology. 2010. PMID: 20638938
-
Global and context-specific transcriptional consequences of oncogenic Fbw7 mutations.Elife. 2022 Feb 28;11:e74338. doi: 10.7554/eLife.74338. Elife. 2022. PMID: 35225231 Free PMC article.
-
Aberrant regulation of FBW7 in cancer.Oncotarget. 2014 Apr 30;5(8):2000-15. doi: 10.18632/oncotarget.1859. Oncotarget. 2014. PMID: 24899581 Free PMC article. Review.
-
The two faces of FBW7 in cancer drug resistance.Bioessays. 2011 Nov;33(11):851-9. doi: 10.1002/bies.201100101. Epub 2011 Aug 30. Bioessays. 2011. PMID: 22006825 Free PMC article. Review.
Cited by
-
Oncogenic mutations in the FBXW7 gene of adult T-cell leukemia patients.Proc Natl Acad Sci U S A. 2016 Jun 14;113(24):6731-6. doi: 10.1073/pnas.1601537113. Epub 2016 May 31. Proc Natl Acad Sci U S A. 2016. PMID: 27247421 Free PMC article.
-
Mutational Status of SMAD4 and FBXW7 Affects Clinical Outcome in TP53-Mutated Metastatic Colorectal Cancer.Cancers (Basel). 2022 Nov 30;14(23):5921. doi: 10.3390/cancers14235921. Cancers (Basel). 2022. PMID: 36497403 Free PMC article.
-
Oncogenic function of SCCRO5/DCUN1D5 requires its Neddylation E3 activity and nuclear localization.Clin Cancer Res. 2014 Jan 15;20(2):372-81. doi: 10.1158/1078-0432.CCR-13-1252. Epub 2013 Nov 5. Clin Cancer Res. 2014. PMID: 24192928 Free PMC article.
-
Tumor suppression by the Fbw7 ubiquitin ligase: mechanisms and opportunities.Cancer Cell. 2014 Oct 13;26(4):455-64. doi: 10.1016/j.ccell.2014.09.013. Cancer Cell. 2014. PMID: 25314076 Free PMC article. Review.
-
Roles of F-box proteins in cancer.Nat Rev Cancer. 2014 Apr;14(4):233-47. doi: 10.1038/nrc3700. Nat Rev Cancer. 2014. PMID: 24658274 Free PMC article. Review.
References
-
- Akhoondi S, et al. 2007. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res. 67:9006–9012 - PubMed
-
- Bauer KD, et al. 1993. Consensus review of the clinical utility of DNA flow cytometry in colorectal cancer. Cytometry 14:486–491 - PubMed
-
- Boivin GP, et al. 2003. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology 124:762–777 - PubMed
-
- Clurman BE, Sheaff RJ, Thress K, Groudine M, Roberts JM. 1996. Turnover of cyclin E by the ubiquitin-proteasome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev. 10:1979–1990 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
