Ingested human insulin inhibits the mosquito NF-κB-dependent immune response to Plasmodium falciparum
- PMID: 22473605
- PMCID: PMC3370580
- DOI: 10.1128/IAI.00024-12
Ingested human insulin inhibits the mosquito NF-κB-dependent immune response to Plasmodium falciparum
Abstract
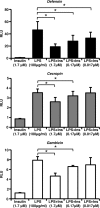
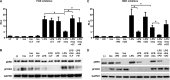
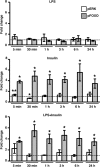
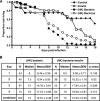
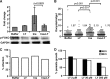
We showed previously that ingested human insulin activates the insulin/IGF-1 signaling pathway in Anopheles stephensi and increases the susceptibility of these mosquitoes to Plasmodium falciparum. In other organisms, insulin can alter immune responsiveness through regulation of NF-κB transcription factors, critical elements for innate immunity that are also central to mosquito immunity. We show here that insulin signaling decreased expression of NF-κB-regulated immune genes in mosquito cells stimulated with either bacterial or malarial soluble products. Further, human insulin suppressed mosquito immunity through sustained phosphatidylinositol 3-kinase activation, since inhibition of this pathway led to decreased parasite development in the mosquito. Together, these data demonstrate that activation of the insulin/IGF-1 signaling pathway by ingested human insulin can alter NF-κB-dependent immunity, and ultimately the susceptibility, of mosquitoes to P. falciparum.
Figures

Similar articles
-
Protein kinase C-dependent signaling controls the midgut epithelial barrier to malaria parasite infection in anopheline mosquitoes.PLoS One. 2013 Oct 11;8(10):e76535. doi: 10.1371/journal.pone.0076535. eCollection 2013. PLoS One. 2013. PMID: 24146884 Free PMC article.
-
Plasmodium falciparum suppresses the host immune response by inducing the synthesis of insulin-like peptides (ILPs) in the mosquito Anopheles stephensi.Dev Comp Immunol. 2015 Nov;53(1):134-44. doi: 10.1016/j.dci.2015.06.012. Epub 2015 Jul 9. Dev Comp Immunol. 2015. PMID: 26165161 Free PMC article.
-
Abscisic acid induces a transient shift in signaling that enhances NF-κB-mediated parasite killing in the midgut of Anopheles stephensi without reducing lifespan or fecundity.Parasit Vectors. 2017 Jul 13;10(1):333. doi: 10.1186/s13071-017-2276-4. Parasit Vectors. 2017. PMID: 28705245 Free PMC article.
-
NF-κB-Like Signaling Pathway REL2 in Immune Defenses of the Malaria Vector Anopheles gambiae.Front Cell Infect Microbiol. 2017 Jun 21;7:258. doi: 10.3389/fcimb.2017.00258. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28680852 Free PMC article. Review.
-
The insulin signaling cascade from nematodes to mammals: insights into innate immunity of Anopheles mosquitoes to malaria parasite infection.Dev Comp Immunol. 2007;31(7):647-56. doi: 10.1016/j.dci.2006.10.005. Epub 2006 Nov 27. Dev Comp Immunol. 2007. PMID: 17161866 Free PMC article. Review.
Cited by
-
Protein kinase C-dependent signaling controls the midgut epithelial barrier to malaria parasite infection in anopheline mosquitoes.PLoS One. 2013 Oct 11;8(10):e76535. doi: 10.1371/journal.pone.0076535. eCollection 2013. PLoS One. 2013. PMID: 24146884 Free PMC article.
-
Sustained activation of Akt elicits mitochondrial dysfunction to block Plasmodium falciparum infection in the mosquito host.PLoS Pathog. 2013 Feb;9(2):e1003180. doi: 10.1371/journal.ppat.1003180. Epub 2013 Feb 28. PLoS Pathog. 2013. PMID: 23468624 Free PMC article.
-
Insulin-like peptide 3 stimulates hemocytes to proliferate in anautogenous and facultatively autogenous mosquitoes.J Exp Biol. 2022 Mar 1;225(5):jeb243460. doi: 10.1242/jeb.243460. Epub 2022 Mar 8. J Exp Biol. 2022. PMID: 35129195 Free PMC article.
-
The Anopheles innate immune system in the defense against malaria infection.J Innate Immun. 2014;6(2):169-81. doi: 10.1159/000353602. Epub 2013 Aug 28. J Innate Immun. 2014. PMID: 23988482 Free PMC article. Review.
-
Human IGF1 regulates midgut oxidative stress and epithelial homeostasis to balance lifespan and Plasmodium falciparum resistance in Anopheles stephensi.PLoS Pathog. 2014 Jun 26;10(6):e1004231. doi: 10.1371/journal.ppat.1004231. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24968248 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

