Effects of hepatitis B virus S protein exposure on sperm membrane integrity and functions
- PMID: 22470450
- PMCID: PMC3314651
- DOI: 10.1371/journal.pone.0033471
Effects of hepatitis B virus S protein exposure on sperm membrane integrity and functions
Abstract
Background: Hepatitis B is a public health problem worldwide. Viral infection can affect a man's fertility, but only scant information about the influence of hepatitis B virus (HBV) infection on sperm quality is available. The purpose of this study was to investigate the effect of hepatitis B virus S protein (HBs) on human sperm membrane integrity and functions.
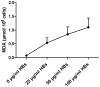
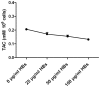
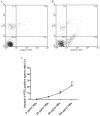
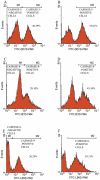
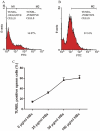
Methods/principal findings: Reactive oxygen species (ROS), lipid peroxidation (LP), total antioxidant capacity (TAC) and phosphatidylserine (PS) externalization were determined. The terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assays and flow cytometric analyses were performed. (1) After 3 h incubation with 25 µg/ml of HBs, the average rates of ROS positive cells, annexin V-positive/propidium iodide (PI)-negative cells, Caspases-3,-8,-9 positive cells and TUNEL-positive cells were significantly increased in the test groups as compared to those in the control groups, while TAC level was decreased when compared with the control. The level of malondialdehyde (MDA) in the sperm cells exposed to 50 µg/ml of HBs for 3 h was significantly higher than that in the control (P<0.05-0.01). (2) HBs increased the MDA levels and the numbers of ROS positive cells, annexin V-positive/PI-negative cells, caspases-3, -8, -9 positive cells and TUNEL-positive cells in a dose-dependent manner. (3) HBs monoclonal antibody (MAb) and N-Acetylcysteine (NAC) reduced the number of ROS-positive sperm cells. (4) HBs decreased the TAC levels in sperm cells in a dose-dependent manner.
Conclusion: HBs exposure could lead to ROS generation, lipid peroxidation, TAC reduction, PS externalization, activation of caspases, and DNA fragmentation, resulting in increased apoptosis of sperm cells and loss of sperm membrane integrity and causing sperm dysfunctions.
Conflict of interest statement
Figures
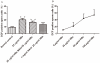
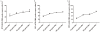
Similar articles
-
Phosphatidylserine externalization in human sperm induced by calcium ionophore A23187: relationship with apoptosis, membrane scrambling and the acrosome reaction.Hum Reprod. 2005 Dec;20(12):3459-68. doi: 10.1093/humrep/dei245. Epub 2005 Aug 19. Hum Reprod. 2005. PMID: 16113043
-
Effects of hepatitis B virus S protein on human sperm function.Hum Reprod. 2009 Jul;24(7):1575-83. doi: 10.1093/humrep/dep050. Epub 2009 Mar 11. Hum Reprod. 2009. PMID: 19279032
-
Hepatitis B virus surface protein induces sperm dysfunction through the activation of a Bcl2/Bax signaling cascade triggering AIF/Endo G-mediated apoptosis.Andrology. 2021 May;9(3):944-955. doi: 10.1111/andr.12965. Epub 2021 Feb 1. Andrology. 2021. PMID: 33382193 Free PMC article.
-
Spontaneous DNA fragmentation in swim-up selected human spermatozoa during long term incubation.J Androl. 2003 Mar-Apr;24(2):253-62. doi: 10.1002/j.1939-4640.2003.tb02670.x. J Androl. 2003. PMID: 12634313
-
Reactive oxygen species and sperm function--in sickness and in health.J Androl. 2012 Nov-Dec;33(6):1096-106. doi: 10.2164/jandrol.112.016535. Epub 2012 Aug 9. J Androl. 2012. PMID: 22879525 Review.
Cited by
-
Hepatitis B virus infection, infertility, and assisted reproduction.J Zhejiang Univ Sci B. 2024 Aug 15;25(8):672-685. doi: 10.1631/jzus.B2300261. J Zhejiang Univ Sci B. 2024. PMID: 39155780 Free PMC article. Review.
-
Update on known and emergent viruses affecting human male genital tract and fertility.Basic Clin Androl. 2024 Mar 14;34(1):6. doi: 10.1186/s12610-024-00222-5. Basic Clin Androl. 2024. PMID: 38486154 Free PMC article. Review.
-
The association between hepatitis B virus and semen quality: a systematic review and meta-analysis.BMC Urol. 2024 Feb 22;24(1):47. doi: 10.1186/s12894-024-01424-9. BMC Urol. 2024. PMID: 38389059 Free PMC article.
-
Astaxanthin Improved the Quality of Hu Ram Semen by Increasing the Antioxidant Capacity and Mitochondrial Potential and Mitigating Free Radicals-Induced Oxidative Damage.Animals (Basel). 2024 Jan 19;14(2):319. doi: 10.3390/ani14020319. Animals (Basel). 2024. PMID: 38275779 Free PMC article.
-
Are sexually transmitted infections associated with male infertility? A systematic review and in-depth evaluation of the evidence and mechanisms of action of 11 pathogens.Arab J Urol. 2023 Jul 5;21(4):216-232. doi: 10.1080/2090598X.2023.2218566. eCollection 2023. Arab J Urol. 2023. PMID: 38178949 Free PMC article. Review.
References
-
- World Health Organization (WHO) Hepatitis B. Fact Sheet WHO/204. (2008) Revised October 2008.
-
- Ganem D. Hepadnaviridae and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology, 3rd edn. Philadelphia: Lippincott-Raven; 1996. pp. 2703–2738.
-
- Scott RM, Snitbhan R, Bancroft WH, Alter HJ, Tingpalapong M. Experimental transmission of hepatitis B virus by semen and saliva. J Infect Dis. 1980;142:67–71. - PubMed
-
- Hadchouel M, Scotto J, Huret JL, Molinie C, Villa E, et al. Presence of HBV DNA in spermatozoa: a possible vertical transmission of HBV via the germ line. J Med Virol. 1985;16:61–66. - PubMed
-
- Lang ZW. Distribution of hepatitis B virus in testicle tissue in patients with hepatitis B infection. Zhonghua Yi Xue Za Zhi. 1993;73:329–331, 379. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

