A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism
- PMID: 22466288
- PMCID: PMC3341994
- DOI: 10.1038/nature10986
A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism
Abstract
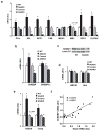
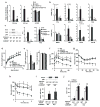
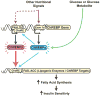
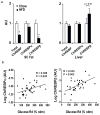
The prevalence of obesity and type 2 diabetes is increasing worldwide and threatens to shorten lifespan. Impaired insulin action in peripheral tissues is a major pathogenic factor. Insulin stimulates glucose uptake in adipose tissue through the GLUT4 (also known as SLC2A4) glucose transporter, and alterations in adipose tissue GLUT4 expression or function regulate systemic insulin sensitivity. Downregulation of human and mouse adipose tissue GLUT4 occurs early in diabetes development. Here we report that adipose tissue GLUT4 regulates the expression of carbohydrate-responsive-element-binding protein (ChREBP; also known as MLXIPL), a transcriptional regulator of lipogenic and glycolytic genes. Furthermore, adipose ChREBP is a major determinant of adipose tissue fatty acid synthesis and systemic insulin sensitivity. We find a new mechanism for glucose regulation of ChREBP: glucose-mediated activation of the canonical ChREBP isoform (ChREBP-α) induces expression of a novel, potent isoform (ChREBP-β) that is transcribed from an alternative promoter. ChREBP-β expression in human adipose tissue predicts insulin sensitivity, indicating that it may be an effective target for treating diabetes.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Comment in
-
Hidden variant of ChREBP in fat links lipogenesis to insulin sensitivity.Cell Metab. 2012 Jun 6;15(6):795-7. doi: 10.1016/j.cmet.2012.05.007. Cell Metab. 2012. PMID: 22682219
-
Good fats: lipidomics approach identify novel regulators of glucose homeostasis.Circ Cardiovasc Genet. 2014 Dec;7(6):965-6. doi: 10.1161/CIRCGENETICS.114.000954. Circ Cardiovasc Genet. 2014. PMID: 25516626 No abstract available.
Similar articles
-
Islet ChREBP-β is increased in diabetes and controls ChREBP-α and glucose-induced gene expression via a negative feedback loop.Mol Metab. 2016 Sep 30;5(12):1208-1215. doi: 10.1016/j.molmet.2016.09.010. eCollection 2016 Dec. Mol Metab. 2016. PMID: 27900263 Free PMC article.
-
Absence of Carbohydrate Response Element Binding Protein in Adipocytes Causes Systemic Insulin Resistance and Impairs Glucose Transport.Cell Rep. 2017 Oct 24;21(4):1021-1035. doi: 10.1016/j.celrep.2017.09.091. Cell Rep. 2017. PMID: 29069585 Free PMC article.
-
Deletion of hepatic carbohydrate response element binding protein (ChREBP) impairs glucose homeostasis and hepatic insulin sensitivity in mice.Mol Metab. 2017 Nov;6(11):1381-1394. doi: 10.1016/j.molmet.2017.07.006. Epub 2017 Jul 18. Mol Metab. 2017. PMID: 29107286 Free PMC article.
-
ChREBP-Mediated Regulation of Lipid Metabolism: Involvement of the Gut Microbiota, Liver, and Adipose Tissue.Front Endocrinol (Lausanne). 2020 Dec 3;11:587189. doi: 10.3389/fendo.2020.587189. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33343508 Free PMC article. Review.
-
The Roles of Carbohydrate Response Element Binding Protein in the Relationship between Carbohydrate Intake and Diseases.Int J Mol Sci. 2021 Nov 8;22(21):12058. doi: 10.3390/ijms222112058. Int J Mol Sci. 2021. PMID: 34769488 Free PMC article. Review.
Cited by
-
Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass.PLoS Biol. 2013;11(2):e1001485. doi: 10.1371/journal.pbio.1001485. Epub 2013 Feb 19. PLoS Biol. 2013. PMID: 23431266 Free PMC article. Clinical Trial.
-
SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways.Sci Rep. 2021 Apr 14;11(1):8177. doi: 10.1038/s41598-021-87759-x. Sci Rep. 2021. PMID: 33854178 Free PMC article.
-
Genome-Wide Analysis of ChREBP Binding Sites on Male Mouse Liver and White Adipose Chromatin.Endocrinology. 2015 Jun;156(6):1982-94. doi: 10.1210/en.2014-1666. Epub 2015 Mar 9. Endocrinology. 2015. PMID: 25751637 Free PMC article.
-
Attenuation of diet-induced obesity and induction of white fat browning with a chemical inhibitor of histone deacetylases.Int J Obes (Lond). 2017 Feb;41(2):289-298. doi: 10.1038/ijo.2016.191. Epub 2016 Oct 28. Int J Obes (Lond). 2017. PMID: 27795551
-
The E3 ligase synoviolin controls body weight and mitochondrial biogenesis through negative regulation of PGC-1β.EMBO J. 2015 Apr 15;34(8):1042-55. doi: 10.15252/embj.201489897. Epub 2015 Feb 19. EMBO J. 2015. PMID: 25698262 Free PMC article.
References
-
- Shepherd PR, Kahn BB. Glucose transporters and insulin action--implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341:248–257. - PubMed
-
- Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. - PubMed
-
- Abel ED, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. - PubMed
-
- Shepherd PR, et al. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem. 1993;268:22243–22246. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- R37 DK43051/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- R01 DK037948-22/DK/NIDDK NIH HHS/United States
- DK037948/DK/NIDDK NIH HHS/United States
- P30 DK046200/DK/NIDDK NIH HHS/United States
- R37 DK043051/DK/NIDDK NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- R01 DK043051/DK/NIDDK NIH HHS/United States
- R01 DK037948/DK/NIDDK NIH HHS/United States
- P30 DK056341-11/DK/NIDDK NIH HHS/United States
- R01 DK098002/DK/NIDDK NIH HHS/United States
- DK056341/DK/NIDDK NIH HHS/United States
- DK057521/DK/NIDDK NIH HHS/United States
- DK046200/DK/NIDDK NIH HHS/United States
- K08 DK076726/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

