Minipig as a potential translatable model for monoclonal antibody pharmacokinetics after intravenous and subcutaneous administration
- PMID: 22453096
- PMCID: PMC3361660
- DOI: 10.4161/mabs.4.2.19387
Minipig as a potential translatable model for monoclonal antibody pharmacokinetics after intravenous and subcutaneous administration
Abstract
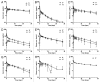
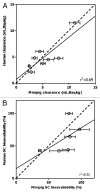
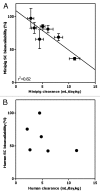
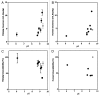
Subcutaneous (SC) delivery is a common route of administration for therapeutic monoclonal antibodies (mAbs) with pharmacokinetic (PK)/pharmacodynamic (PD) properties requiring long-term or frequent drug administration. An ideal in vivo preclinical model for predicting human PK following SC administration may be one in which the skin and overall physiological characteristics are similar to that of humans. In this study, the PK properties of a series of therapeutic mAbs following intravenous (IV) and SC administration in Göttingen minipigs were compared with data obtained previously from humans. The present studies demonstrated: (1) minipig is predictive of human linear clearance; (2) the SC bioavailabilities in minipigs are weakly correlated with those in human; (3) minipig mAb SC absorption rates are generally higher than those in human and (4) the SC bioavailability appears to correlate with systemic clearance in minipigs. Given the important role of the neonatal Fc-receptor (FcRn) in the PK of mAbs, the in vitro binding affinities of these IgGs against porcine, human and cynomolgus monkey FcRn were tested. The result showed comparable FcRn binding affinities across species. Further, mAbs with higher isoelectric point tended to have faster systemic clearance and lower SC bioavailability in both minipig and human. Taken together, these data lend increased support for the use of the minipig as an alternative predictive model for human IV and SC PK of mAbs.
Keywords: animal model; mAb IgG; minipig; neonatal Fc receptor (FcRn); pharmacokinetics; subcutaneous bioavailability.
Figures
Similar articles
-
Clearance of therapeutic antibody glycoforms after subcutaneous and intravenous injection in a porcine model.MAbs. 2022 Jan-Dec;14(1):2145929. doi: 10.1080/19420862.2022.2145929. MAbs. 2022. PMID: 36383465 Free PMC article.
-
Hematopoietic cells as site of first-pass catabolism after subcutaneous dosing and contributors to systemic clearance of a monoclonal antibody in mice.MAbs. 2018 Jul;10(5):803-813. doi: 10.1080/19420862.2018.1458808. Epub 2018 May 9. MAbs. 2018. PMID: 29621428 Free PMC article.
-
In vitro model for predicting bioavailability of subcutaneously injected monoclonal antibodies.J Control Release. 2018 Mar 10;273:13-20. doi: 10.1016/j.jconrel.2018.01.015. Epub 2018 Feb 6. J Control Release. 2018. PMID: 29355621
-
Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins.Protein Cell. 2018 Jan;9(1):15-32. doi: 10.1007/s13238-017-0408-4. Epub 2017 Apr 19. Protein Cell. 2018. PMID: 28421387 Free PMC article. Review.
-
Subcutaneous delivery of monoclonal antibodies: How do we get there?J Control Release. 2018 Sep 28;286:301-314. doi: 10.1016/j.jconrel.2018.08.001. Epub 2018 Aug 2. J Control Release. 2018. PMID: 30077735 Review.
Cited by
-
Clearance of therapeutic antibody glycoforms after subcutaneous and intravenous injection in a porcine model.MAbs. 2022 Jan-Dec;14(1):2145929. doi: 10.1080/19420862.2022.2145929. MAbs. 2022. PMID: 36383465 Free PMC article.
-
A PBPK workflow for first-in-human dose selection of a subcutaneously administered pegylated peptide.J Pharmacokinet Pharmacodyn. 2015 Apr;42(2):135-50. doi: 10.1007/s10928-015-9406-4. Epub 2015 Feb 4. J Pharmacokinet Pharmacodyn. 2015. PMID: 25650156
-
A Physiologically Based Pharmacokinetic Model Relates the Subcutaneous Bioavailability of Monoclonal Antibodies to the Saturation of FcRn-Mediated Recycling in Injection-Site-Draining Lymph Nodes.Antibodies (Basel). 2024 Aug 15;13(3):70. doi: 10.3390/antib13030070. Antibodies (Basel). 2024. PMID: 39189241 Free PMC article.
-
Tau antibody chimerization alters its charge and binding, thereby reducing its cellular uptake and efficacy.EBioMedicine. 2019 Apr;42:157-173. doi: 10.1016/j.ebiom.2019.03.033. Epub 2019 Mar 22. EBioMedicine. 2019. PMID: 30910484 Free PMC article.
-
Pharmacokinetic and Pharmacodynamic Properties of Drug Delivery Systems.J Pharmacol Exp Ther. 2019 Sep;370(3):570-580. doi: 10.1124/jpet.119.257113. Epub 2019 Mar 5. J Pharmacol Exp Ther. 2019. PMID: 30837281 Free PMC article. Review.
References
-
- Green MD, Hartsough M. Preclinical Safety Evaluation of Biopharmaceuticals. John Wiley & Sons, Inc.; 2007. The Role of Pharmacokinetics and Pharmacodynamics in Selecting a Relevant Species; pp. 277–291.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
