Roles of N-acetylglucosaminyltransferase III in epithelial-to-mesenchymal transition induced by transforming growth factor β1 (TGF-β1) in epithelial cell lines
- PMID: 22451656
- PMCID: PMC3351319
- DOI: 10.1074/jbc.M111.262154
Roles of N-acetylglucosaminyltransferase III in epithelial-to-mesenchymal transition induced by transforming growth factor β1 (TGF-β1) in epithelial cell lines
Abstract
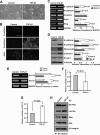
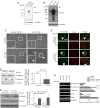
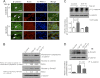
The epithelial-to-mesenchymal transition (EMT) plays crucial roles in embryonic development, wound healing, tissue repair, and cancer progression. Results of this study show how transforming growth factor β1 (TGF-β1) down-regulates expression of N-acetylglucosaminyltransferase III (GnT-III) during EMT-like changes. Treatment with TGF-β1 resulted in a decrease in E-cadherin expression and GnT-III expression, as well as its product, the bisected N-glycans, which was confirmed by erythro-agglutinating phytohemagglutinin lectin blot and HPLC analysis in human MCF-10A and mouse GE11 cells. In contrast with GnT-III, the expression of N-acetylglucosaminyltransferase V was slightly enhanced by TGF-β1 treatment. Changes in the N-glycan patterns on α3β1 integrin, one of the target proteins for GnT-III, were also confirmed by lectin blot analysis. To understand the roles of GnT-III expression in EMT-like changes, the MCF-10A cell was stably transfected with GnT-III. It is of particular interest that overexpression of GnT-III influenced EMT-like changes induced by TGF-β1, which was confirmed by cell morphological changes of phase contrast, immunochemical staining patterns of E-cadherin, and actin. In addition, GnT-III modified E-cadherin, which served to prolong E-cadherin turnover on the cell surface examined by biotinylation and pulse-chase experiments. GnT-III expression consistently inhibited β-catenin translocation from cell-cell contact into the cytoplasm and nucleus. Furthermore, the transwell assay showed that GnT-III expression suppressed TGF-β1-induced cell motility. Taken together, these observations are the first to clearly demonstrate that GnT-III affects cell properties, which in turn influence EMT-like changes, and to explain a molecular mechanism for the inhibitory effects of GnT-III on cancer metastasis.
Figures


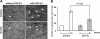
Similar articles
-
Wnt/beta-catenin signaling down-regulates N-acetylglucosaminyltransferase III expression: the implications of two mutually exclusive pathways for regulation.J Biol Chem. 2011 Feb 11;286(6):4310-8. doi: 10.1074/jbc.M110.182576. Epub 2010 Nov 29. J Biol Chem. 2011. PMID: 21115490 Free PMC article.
-
Specific N-glycan alterations are coupled in epithelial-mesenchymal transition induced by EGF in GE11 epithelial cells.Cell Biol Int. 2017 Feb;41(2):124-133. doi: 10.1002/cbin.10707. Epub 2016 Dec 29. Cell Biol Int. 2017. PMID: 27888538
-
Transforming growth factor-β1 induces epithelial-mesenchymal transition and integrin α3β1-mediated cell migration of HSC-4 human squamous cell carcinoma cells through Slug.J Biochem. 2013 Mar;153(3):303-15. doi: 10.1093/jb/mvs144. Epub 2012 Dec 17. J Biochem. 2013. PMID: 23248240
-
True significance of N-acetylglucosaminyltransferases GnT-III, V and α1,6 fucosyltransferase in epithelial-mesenchymal transition and cancer.Mol Aspects Med. 2021 Jun;79:100905. doi: 10.1016/j.mam.2020.100905. Epub 2020 Sep 30. Mol Aspects Med. 2021. PMID: 33010941 Review.
-
Bioinformatic approaches to augment study of epithelial-to-mesenchymal transition in lung cancer.Physiol Genomics. 2014 Oct 1;46(19):699-724. doi: 10.1152/physiolgenomics.00062.2014. Epub 2014 Aug 5. Physiol Genomics. 2014. PMID: 25096367 Free PMC article. Review.
Cited by
-
Evidence for and against epithelial-to-mesenchymal transition in the liver.Am J Physiol Gastrointest Liver Physiol. 2013 Dec;305(12):G881-90. doi: 10.1152/ajpgi.00289.2013. Epub 2013 Oct 24. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 24157970 Free PMC article.
-
RNA-seq analysis of glycosylation related gene expression in STZ-induced diabetic rat kidney inner medulla.Front Physiol. 2015 Oct 1;6:274. doi: 10.3389/fphys.2015.00274. eCollection 2015. Front Physiol. 2015. PMID: 26483702 Free PMC article.
-
Specific N-glycan alterations are coupled in EMT induced by different density cultivation of MCF 10A epithelial cells.Glycoconj J. 2017 Apr;34(2):219-227. doi: 10.1007/s10719-016-9754-3. Epub 2016 Dec 29. Glycoconj J. 2017. PMID: 28035583
-
Enzymes for N-Glycan Branching and Their Genetic and Nongenetic Regulation in Cancer.Biomolecules. 2016 Apr 28;6(2):25. doi: 10.3390/biom6020025. Biomolecules. 2016. PMID: 27136596 Free PMC article. Review.
-
Modulation of N-glycosylation by mesalamine facilitates membranous E-cadherin expression in colon epithelial cells.Biochem Pharmacol. 2014 Jan 15;87(2):312-20. doi: 10.1016/j.bcp.2013.10.021. Epub 2013 Oct 30. Biochem Pharmacol. 2014. PMID: 24184502 Free PMC article.
References
-
- Huber M. A., Kraut N., Beug H. (2005) Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17, 548–558 - PubMed
-
- Thiery J. P., Sleeman J. P. (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7, 131–142 - PubMed
-
- Prall F. (2007) Tumor budding in colorectal carcinoma. Histopathology 50, 151–162 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

