Resolvin E2 formation and impact in inflammation resolution
- PMID: 22450811
- PMCID: PMC3331964
- DOI: 10.4049/jimmunol.1103652
Resolvin E2 formation and impact in inflammation resolution
Abstract
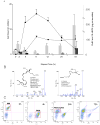
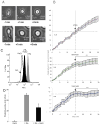
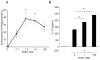
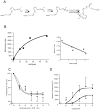
Acute inflammation and its resolution are essential processes for tissue protection and homeostasis. In this context, specialized proresolving mediators derived from polyunsaturated fatty acids are of interest. In this study, we report that resolvin E2 (RvE2) from eicosapentaenoic acid is endogenously produced during self-limited murine peritonitis in both the initiation and resolution phases. RvE2 (1-10 nM) carries potent leukocyte-directed actions that include: 1) regulating chemotaxis of human neutrophils; and 2) enhancing phagocytosis and anti-inflammatory cytokine production. These actions appear to be mediated by leukocyte G-protein-coupled receptors as preparation of labeled RvE2 gave direct evidence for specific binding of radiolabeled RvE2 to neutrophils (K(d) 24.7 ± 10.1 nM) and resolvin E1 activation of recombinant G-protein-coupled receptors was assessed. In addition to the murine inflammatory milieu, RvE2 was also identified in plasma from healthy human subjects. RvE2 rapidly downregulated surface expression of human leukocyte integrins in whole blood and dampened responses to platelet-activating factor. Together, these results indicate that RvE2 can stimulate host-protective actions throughout initiation and resolution in the innate inflammatory responses.
Conflict of interest statement
CNS is an inventor on patents [resolvins] assigned to BWH and licensed to Resolvyx Pharmaceuticals. CNS is a scientific founder of Resolvyx Pharmaceuticals and owns equity in the company. CNS’ interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Figures
Similar articles
-
Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis.Chem Biol. 2006 Nov;13(11):1193-202. doi: 10.1016/j.chembiol.2006.09.011. Chem Biol. 2006. PMID: 17114001
-
Resolvin E1 metabolome in local inactivation during inflammation-resolution.J Immunol. 2008 Mar 1;180(5):3512-9. doi: 10.4049/jimmunol.180.5.3512. J Immunol. 2008. PMID: 18292578
-
Neutrophil Resolvin E1 Receptor Expression and Function in Type 2 Diabetes.J Immunol. 2017 Jan 15;198(2):718-728. doi: 10.4049/jimmunol.1601543. Epub 2016 Dec 19. J Immunol. 2017. PMID: 27994073 Free PMC article.
-
The role of polyunsaturated ω-3 fatty acid eicosapentaenoic acid-derived resolvin E1 (RvE1) in bone preservation.Crit Rev Immunol. 2014;34(4):347-57. doi: 10.1615/critrevimmunol.2014009982. Crit Rev Immunol. 2014. PMID: 24941160 Free PMC article. Review.
-
Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis.Prostaglandins Other Lipid Mediat. 2004 Apr;73(3-4):155-72. doi: 10.1016/j.prostaglandins.2004.03.005. Prostaglandins Other Lipid Mediat. 2004. PMID: 15290791 Review.
Cited by
-
Advanced Age Alters Monocyte and Macrophage Responses.Antioxid Redox Signal. 2016 Nov 20;25(15):805-815. doi: 10.1089/ars.2016.6691. Epub 2016 Aug 3. Antioxid Redox Signal. 2016. PMID: 27357201 Free PMC article. Review.
-
Specialized Pro-Resolving Lipid Mediators: New Therapeutic Approaches for Vascular Remodeling.Int J Mol Sci. 2022 Mar 25;23(7):3592. doi: 10.3390/ijms23073592. Int J Mol Sci. 2022. PMID: 35408952 Free PMC article. Review.
-
Anti-Inflammatory Function of Fatty Acids and Involvement of Their Metabolites in the Resolution of Inflammation in Chronic Obstructive Pulmonary Disease.Int J Mol Sci. 2021 Nov 26;22(23):12803. doi: 10.3390/ijms222312803. Int J Mol Sci. 2021. PMID: 34884621 Free PMC article. Review.
-
Increased tissue levels of omega-3 polyunsaturated fatty acids prevents pathological preterm birth.Sci Rep. 2013 Nov 1;3:3113. doi: 10.1038/srep03113. Sci Rep. 2013. PMID: 24177907 Free PMC article.
-
Specialized Pro-Resolving Mediators Mitigate Cancer-Related Inflammation: Role of Tumor-Associated Macrophages and Therapeutic Opportunities.Front Immunol. 2021 Jun 30;12:702785. doi: 10.3389/fimmu.2021.702785. eCollection 2021. Front Immunol. 2021. PMID: 34276698 Free PMC article. Review.
References
-
- Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS. Robbins and Cotran pathologic basis of disease. Elsevier Saunders; Philadelphia: 2005.
-
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. - PubMed
-
- Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213–220. - PubMed
-
- Stoll G, Kleinschnitz C, Nieswandt B. Combating innate inflammation: a new paradigm for acute treatment of stroke? Ann N Y Acad Sci. 2010;1207:149–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

